23
TITAN
- A Phase 3 Randomized, Placebo-controlled, Double-blind Study of Apalutamide Plus
Androgen Deprivation Therapy (ADT) Versus ADT in Subjects With Metastatic Hormone-
sensitive Prostate Cancer (mHSPC)
•
STRATIFICATION: Volume of Disease (high vs. low); Use of Bone Targeted Agents, Co-Morbidities (0-1
vs. 2-3), Docetaxel Use
•
SPONSOR: Janssen
•
PRIMARY COMPLETION DATE: November 2020
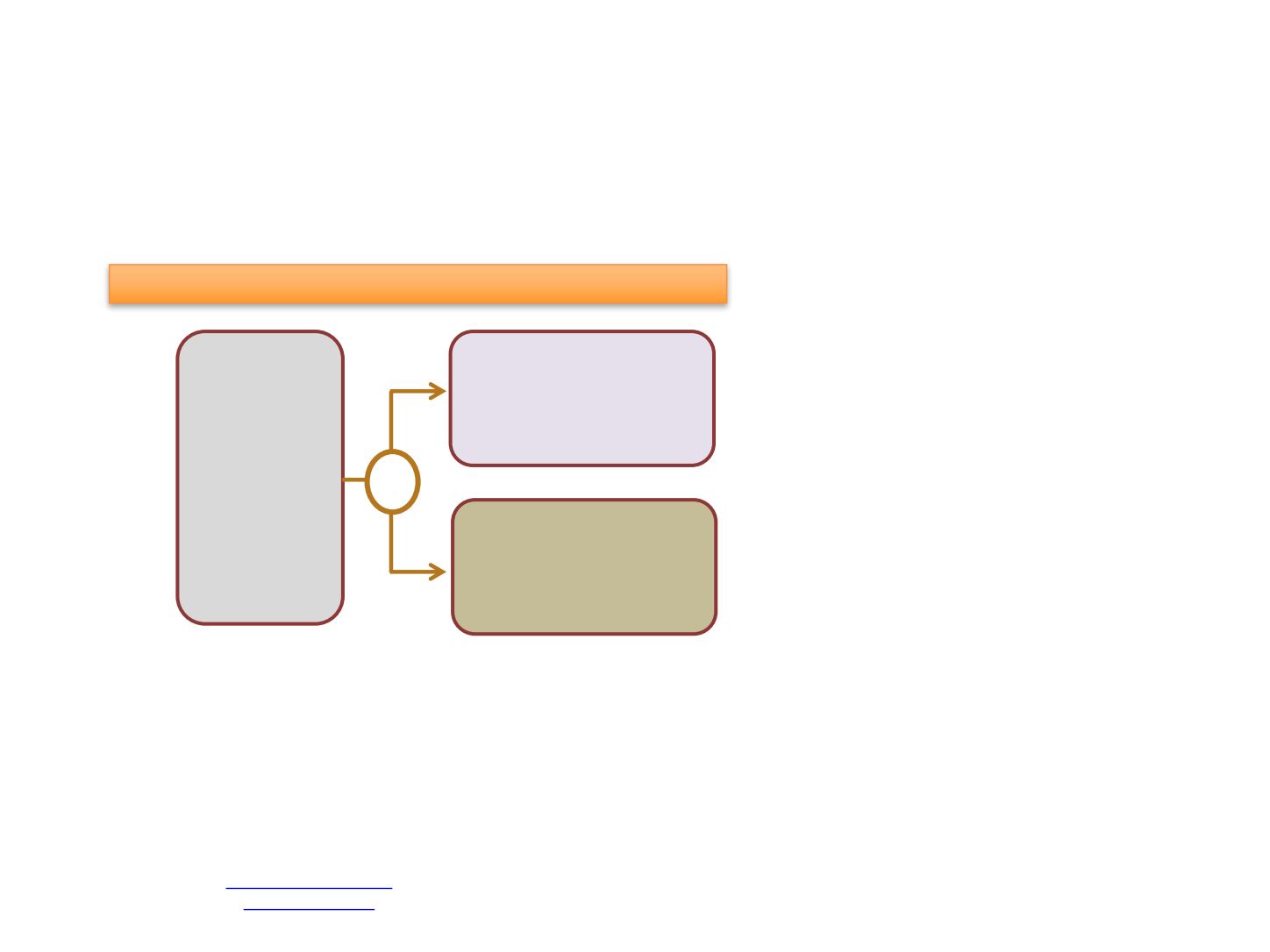
Phase 3, Double-blind, Placebo-Controlled Randomized Study
Positive
bone/CT/MRI
scan, ECOG 0-1,
prior doce use if
stable disease,
1 prior rad tx, ≤6
months ADT,
localized tx if ≥
1 year
randomization
N=1052
ADT
+
Apalutamide
ADT
+
Placebo
R
Primary Objective
-
rPFS and OS
Secondary Objectives
-
Time to Pain
progression
-
Time to SRE
-
Time to Chronic Opioid
Use
-
Time to Initiation of
Cytotoxic
Chemotherapy
TITAN
www.clinicaltrials.gov(NCT02489318).








