24
ARASENS
- A Randomized, Double-blind, Placebo Controlled Phase III Study of Darolutamide (ODM-201)
Versus Placebo in Addition to Standard Androgen Deprivation Therapy and Docetaxel in Patients With
Metastatic Hormone Sensitive Prostate Cancer
•
STRATIFICATION: Unknown
•
SPONSOR: Bayer
•
PRIMARY COMPLETION DATE: August 2022
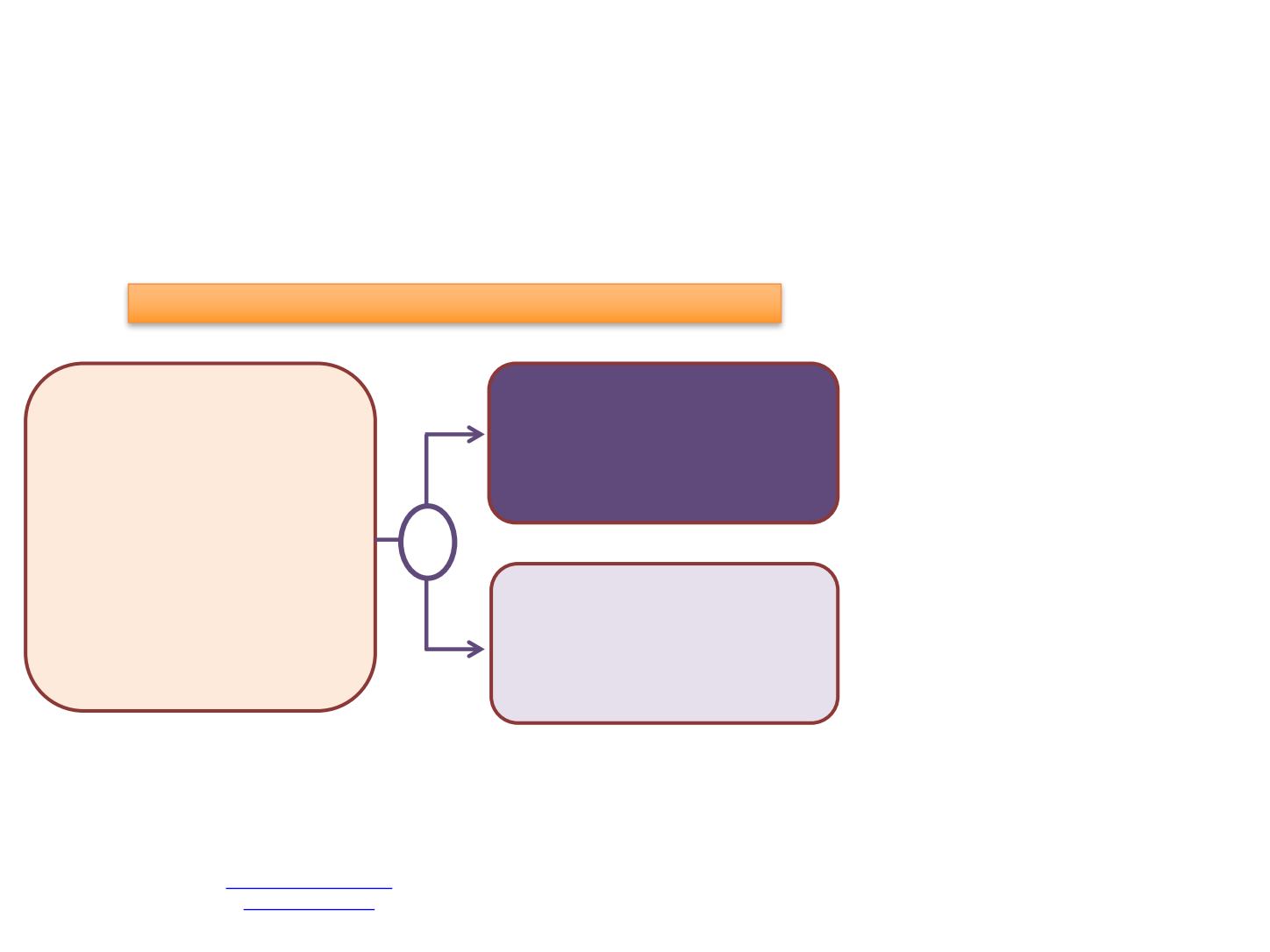
Phase 3, Double-blind, Placebo-Controlled Randomized Study
Metastatic PC (could have rec'd ADT
±
1st gen anti-androgen within 12
weeks randomization), ECOG 0-1,
Adequate hematologic, hepatic &
renal function
N=1303
ADT +
Docetaxel
Darolutamide
(ODM-021)
Placebo
R
Primary Objective
-
OS
Secondary Objectives
-
Time to CRPC
-
Time to Initiation of Subsequent
Antineoplastic Therapy
-
Symptomatic Skeletal Event
Free Survival
-
Time to First Symptomatic
Skeletal Event
-
Time to Opioid Use
-
Time Pain Progression
-
Time to Worsening of Physical
Symptoms of Disease
-
Safety
ARASENS
www.clinicaltrials.gov(NCT02799602).








