25
S1216
- A Phase III Randomized Trial Comparing Androgen Deprivation Therapy + TAK-700 With Androgen
Deprivation Therapy + Bicalutamide in Patients With Newly Diagnosed Metastatic Sensitive Prostate Cancer
•
STRATIFICATION: Unknown
•
SPONSOR: SWOG PI- Neeraj Agarwal (Utah)
•
PRIMARY COMPLETION DATE: March 2022
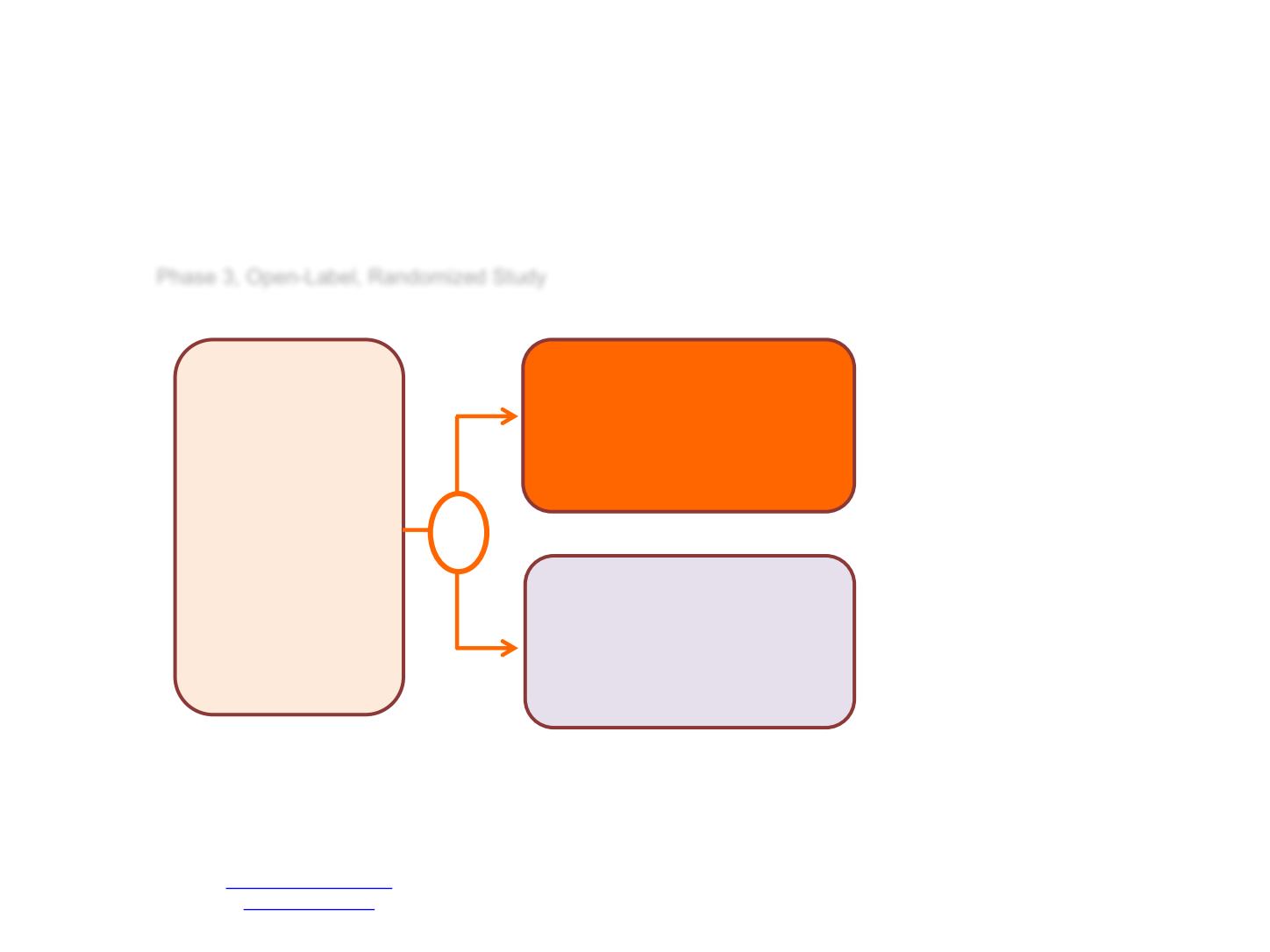
Phase 3, Open-Label, Randomized Study
Metastatic PC, nl
testosterone, PSA ≥ 2,
DEXA scan w/in 2 yrs,
ECG + LVEF WNL,
Adequate hematologic,
hepatic & renal
function, Zubrod PS 0-3
(3 bone pain only)
N=1313
ADT
TAK-700
Bicalutamide
R
Primary Objective
-
OS
Secondary Objectives
-
PFS
-
PSA response at 7
months (<0.2 vs 0.2-4
vs. >4 ng/ml)
-
Safety
-
Long-term Survival after
10 years of Follow-up
S1216
www.clinicaltrials.gov(NCT01809691).








