1. Kudo M
et al
.
Lancet
. 2018;pii:S0140-6736(18)30207–1.
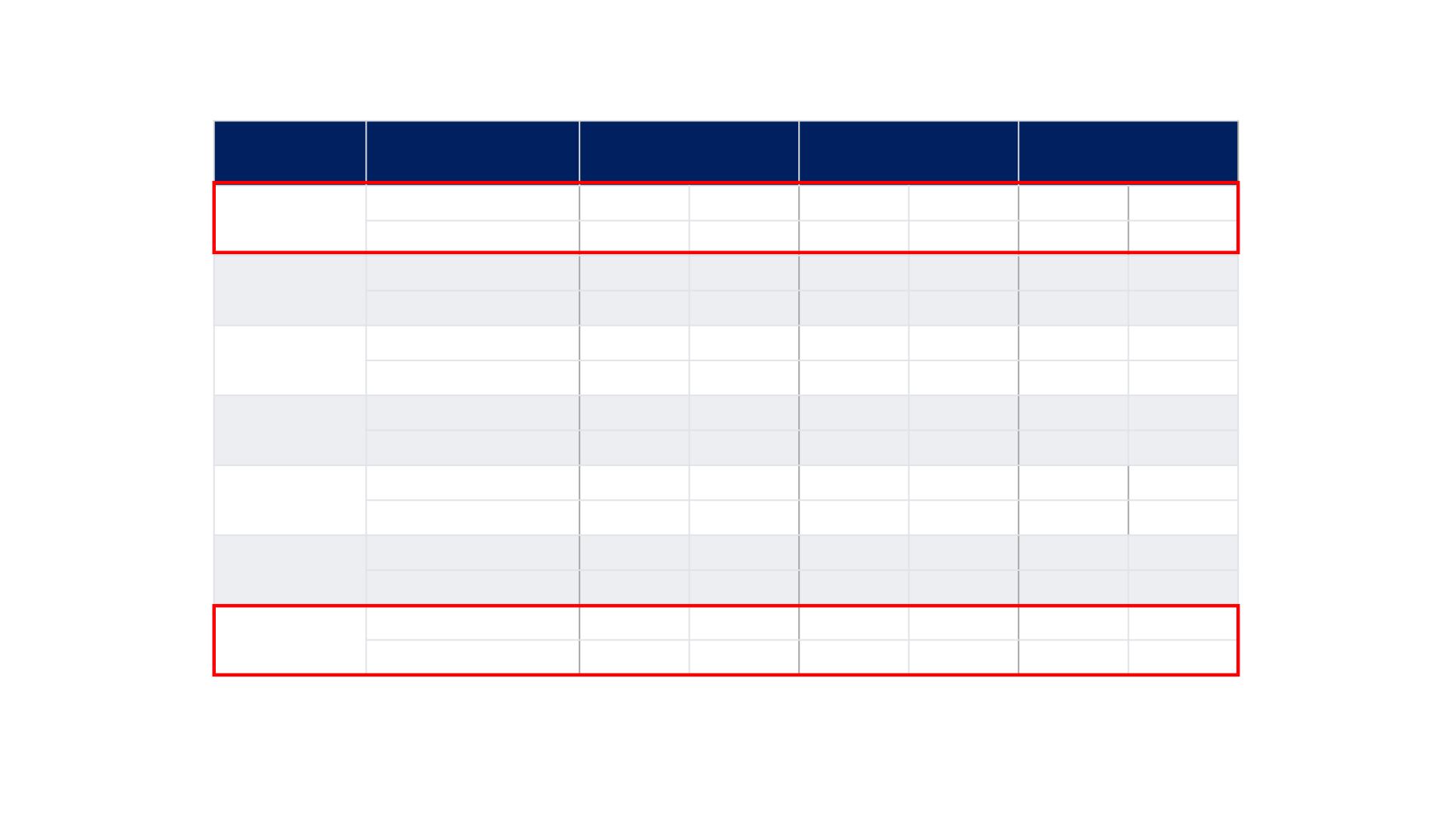
REFLECT TRIAL: Patient Characteristics
*Based on masked independent imaging review.
BCLC = Barcelona Clinic Liver Cancer; ECOG-PS = Eastern Cooperative Oncology Group Performance Status; EHS = extrahepatic spread;
MVI = macroscopic portal vein invasion.
Characteristic
Category
Lenvatinib
(N=478)
Sorafenib
(N=476)
Total
(N=954)
Child-Pugh
class, n, %
A
475
99%
471
99%
946
99%
B
3
1%
5
1%
8
1%
MVI, n, %
Yes
109
23%
90
19%
199
21%
No
369
77%
386
81%
755
79%
EHS, n, %
Yes
291
61%
295
62%
586
61%
No
187
39%
181
38%
368
39%
ECOG-PS, n, %
0
304
64%
301
63%
605
63%
1
174
36%
175
37%
349
37%
MVI, EHS, or
both, n, %
Yes
329
69%
336
71%
665
70%
No
149
31%
140
29%
289
30%
Underlying
cirrhosis*, n, %
Yes
356
74%
364
76%
720
75%
No
122
26%
112
24%
234
25%
BCLC stage, n,
%
B
(intermediate stage)
104
22%
92
19%
196
21%
C
(advanced stage)
374
78%
384
81%
758
79%








