REFLECT TRIAL: Statistical Analysis
CI = confidence interval; FAS = full analysis set/intent to treat; HR = hazard ratio; OS = overall survival; PPS = per protocol set.
§
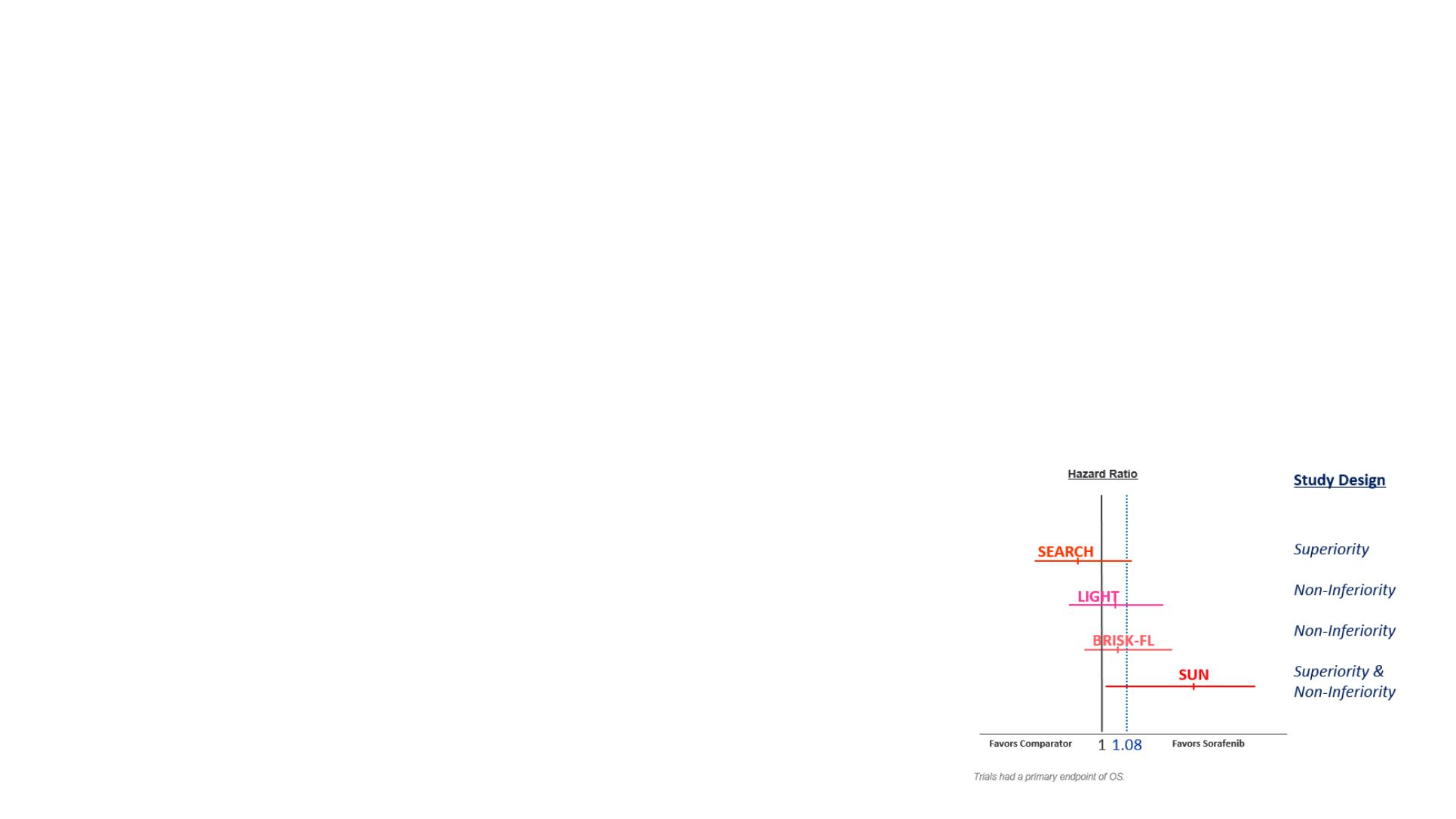
By this method the trial would be successful if the upper limit of the 95% CI for the HR
lies below the non-inferiority margin of 1.08
Ø
Non-inferiority of lenvatinib to sorafenib is inferred – a better than 60% retention of
sorafenib effect by lenvatinib is demonstrated
Ø
Superiority of lenvatinib to putative placebo effect is also demonstrated
§
All analyses of secondary efficacy endpoints were performed based on the FAS
as the primary analysis set and the PPS as the secondary analysis set
§
After non-inferiority was declared, secondary efficacy endpoints were tested
Ø
Sequential method.
1. Data on file LEN-023.; 2. Kudo M et al. Lancet. 2018;pii:S0140-6736(18)30207–1








