1.
Kudo M
et al
.
Lancet
. 2018;pii:S0140-6736(18)30207–1
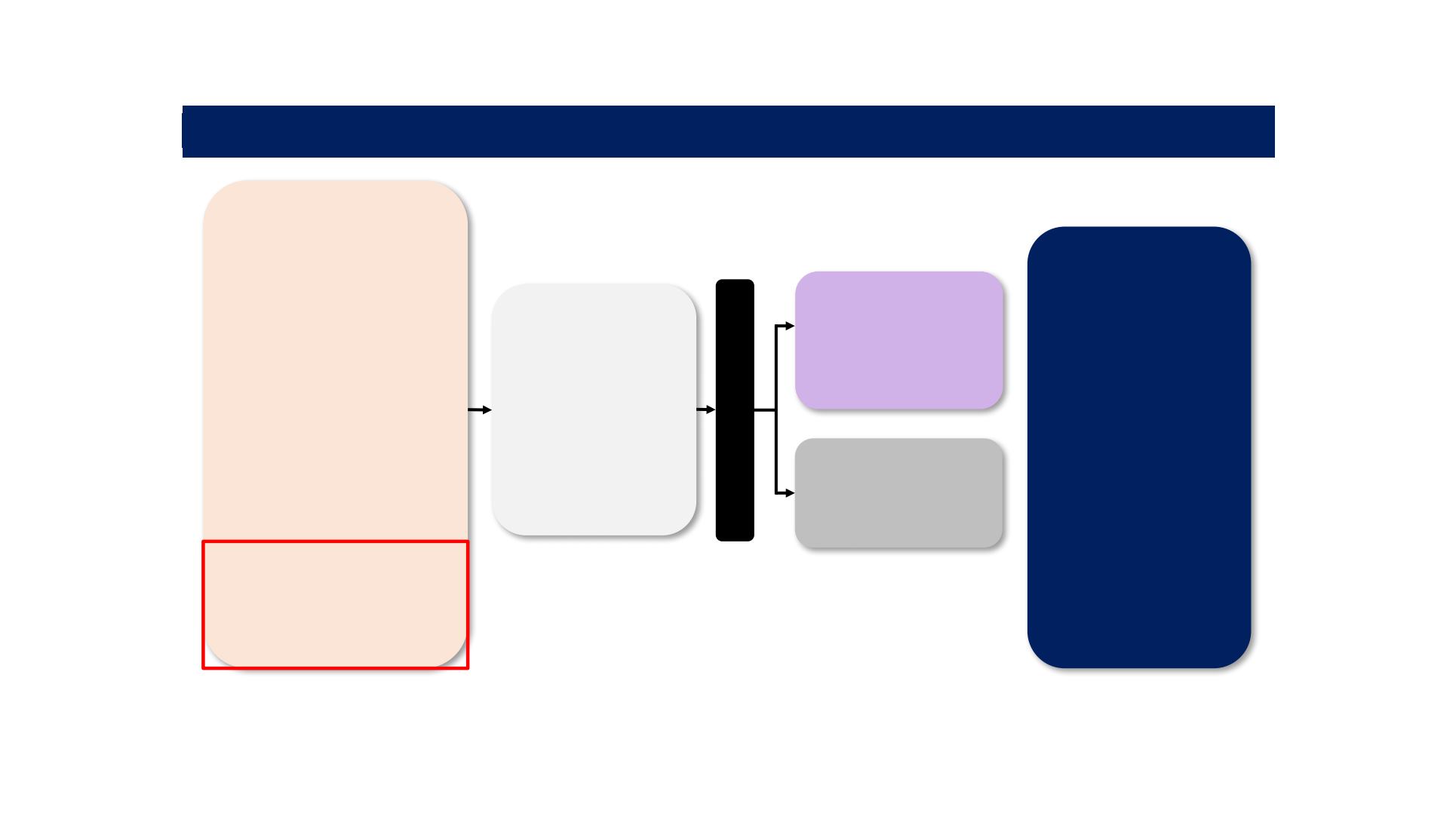
REFLECT TRIAL: Study Schema
BCLC = Barcelona Clinic Liver Cancer; BW = body weight; ECOG-PS = Eastern Cooperative Oncology Group Performance Status; EHS = extrahepatic spread;
HCC = hepatocellular carcinoma; MVI = macroscopic portal vein invasion; mRECIST = modified Response Evaluation Criteria In Solid Tumours; ORR = objective response rate; OS =
overall survival; PFS = progression-free survival; PK = pharmacokinetic; RECIST = Response Evaluation Criteria in Solid Tumours;
TACE = trans-catheter arterial chemoembolisation; TTP = time to progression.
Global, randomised, open-label, phase 3 non-inferiority study
Stratification
§
Region:
(Asia-Pacific or
Western)
§
MVI and/or EHS:
(yes or no)
§
ECOG-PS: (0 or 1)
§
Body weight:
(<60 kg or ≥60 kg)
§
Primary
objective:
‒ OS
§
Secondary
objectives:
‒ PFS
‒ TTP
‒ ORR
‒ Quality of life
‒ PK lenvatinib
exposure
parameters
*
Tumour assessments were
performed according to
mRECIST by the investigator
Patients with
unresectable HCC
(N=954)
§
No prior systemic therapy
for unresectable HCC
§
≥ 1 measurable target lesion
per mRECIST
§
BCLC-B (not applicable for
TACE) or BCLC-C
§
Child-Pugh A
§
ECOG-PS ≤1
§
Adequate organ function
§
Patients with ≥50% liver
occupation, clear bile duct
invasion, or portal vein
invasion at the main portal
vein were excluded
Lenvatinib
(N=478)
8 mg (BW <60 kg) or
12 mg (BW ≥60 kg)
once daily
Sorafenib
(n = 476)
400 mg
twice daily
Randomisation 1:1








