1. Kudo M et al. Lancet. 2018;pii:S0140-6736(18)30207–1
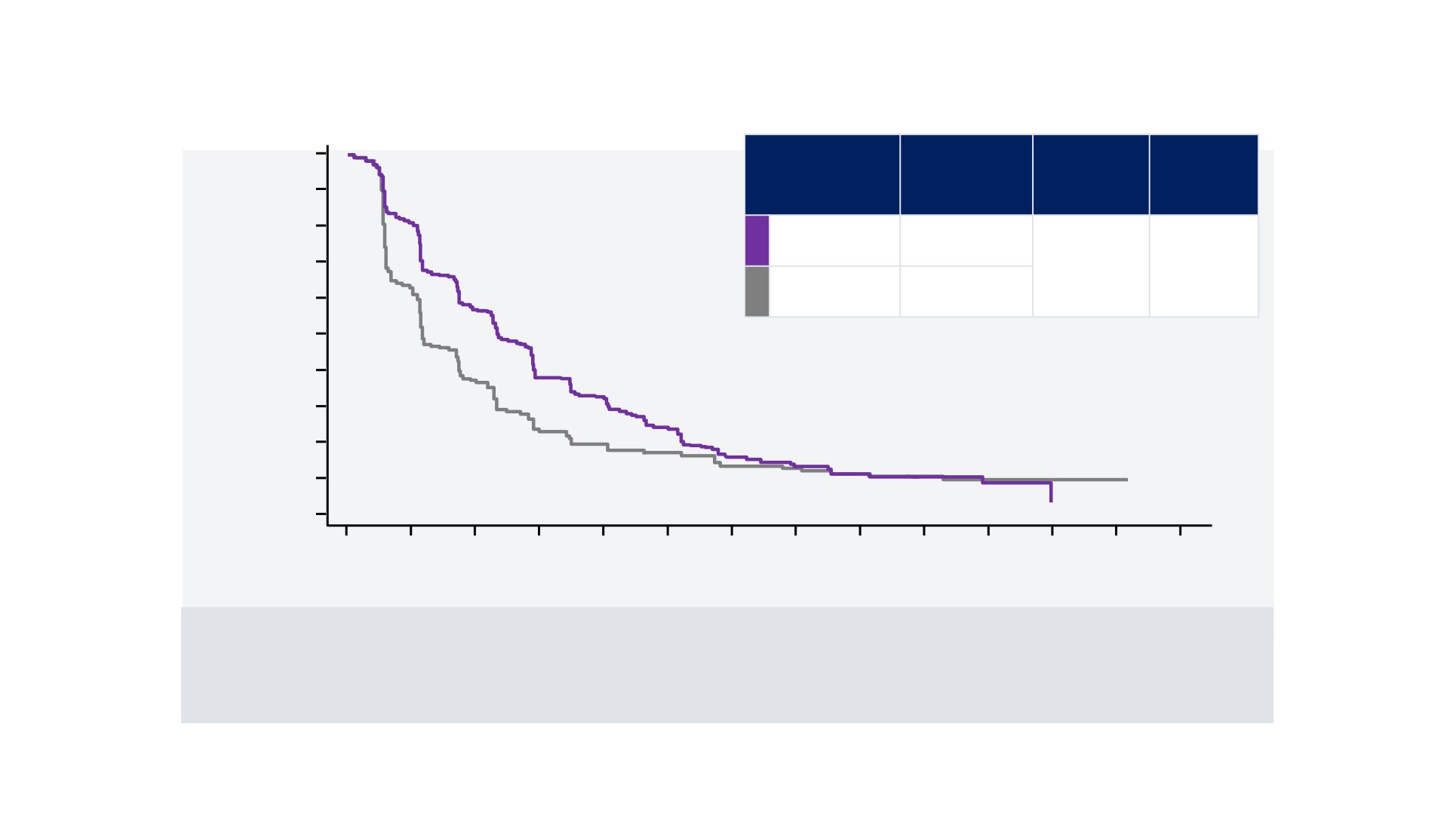
REFLECT TRIAL
Secondary Endpoint: Kaplan-Meier Estimate of PFS by
mRECIST
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
Probability
Time (months)
0
3
6
9
12 15 18 21 24 27 30 33 36 39
+ +
++ + + + +++ +++ ++++ ++ ++++++
+
+++
+
++
+
+
+ +
+++
+
+
+
+
+++
+
+
+
+
+++++ +++++++
+++
+++++
+
++++++
++++
Number of patients at risk:
Lenvatinib
478 345 223 172 106 69 44 28 14 9 4 2 0 0
Sorafenib
476 262 140 94 56 41 33 22 14 9 4 2 2 0
Log rank test: p<0.00001
CI = confidence interval; HR = hazard ratio; mRECIST = modified Response Evaluation Criteria in Solid Tumours; PFS = progression-free survival.
PFS, months
(95% CI)
HR
(95% CI)
Log-rank
test:
p-value
Lenvatinib
7.4
(6.9, 8.8)
0.66
(0.57, 0.77)
<0.0001
Sorafenib
3.7
(3.6, 4.6)








