1. Kudo M
et al
.
Lancet
. 2018;pii:S0140-6736(18)30207–1.
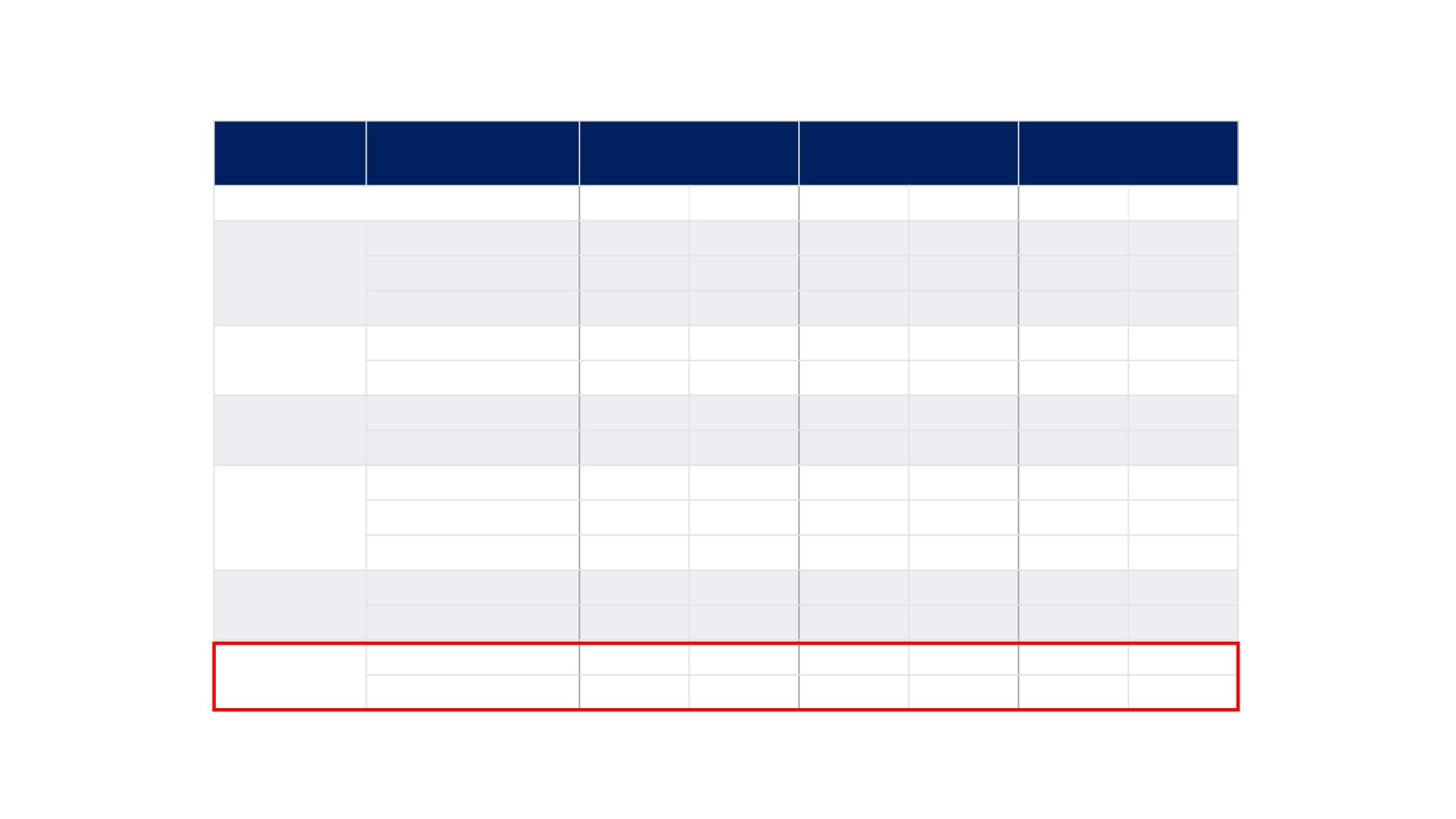
REFLECT TRIAL: Patient Characteristics
Characteristic
Category
Lenvatinib
(N=478)
Sorafenib
(N=476)
Total
(N=954)
Age (years), median, range
63.0
20–88
62.0
22–88
62.0
20–88
Age group
(years), n, %
<65
270
56%
283
59%
553
58%
≥65 to <75
150
31%
126
26%
276
30%
≥75
58
12%
67
14%
125
13%
Sex, n, %
Male
405
85%
401
84%
806
84%
Female
73
15%
75
16%
148
16%
Region, n, %
Western
157
33%
157
33%
314
33%
Asia-Pacific
321
67%
319
67%
640
67%
Race, n, %
White
135
28%
141
30%
276
29%
Asian
334
70%
326
68%
660
69%
Other
9
2%
9
2%
18
2%
Body weight,
n, %
<60 kg
153
32%
146
31%
299
31%
≥60 kg
325
68%
330
69%
655
69%
ECOG-PS, n, %
0
304
64%
301
63%
605
63%
1
174
36%
175
37%
349
37%
ECOG-PS = Eastern Cooperative Oncology Group Performance Status.








