1. Kudo M
et al
.
Lancet
. 2018;pii:S0140-6736(18)30207–1 ; Supplementary Appendix
REFLECT TRIAL
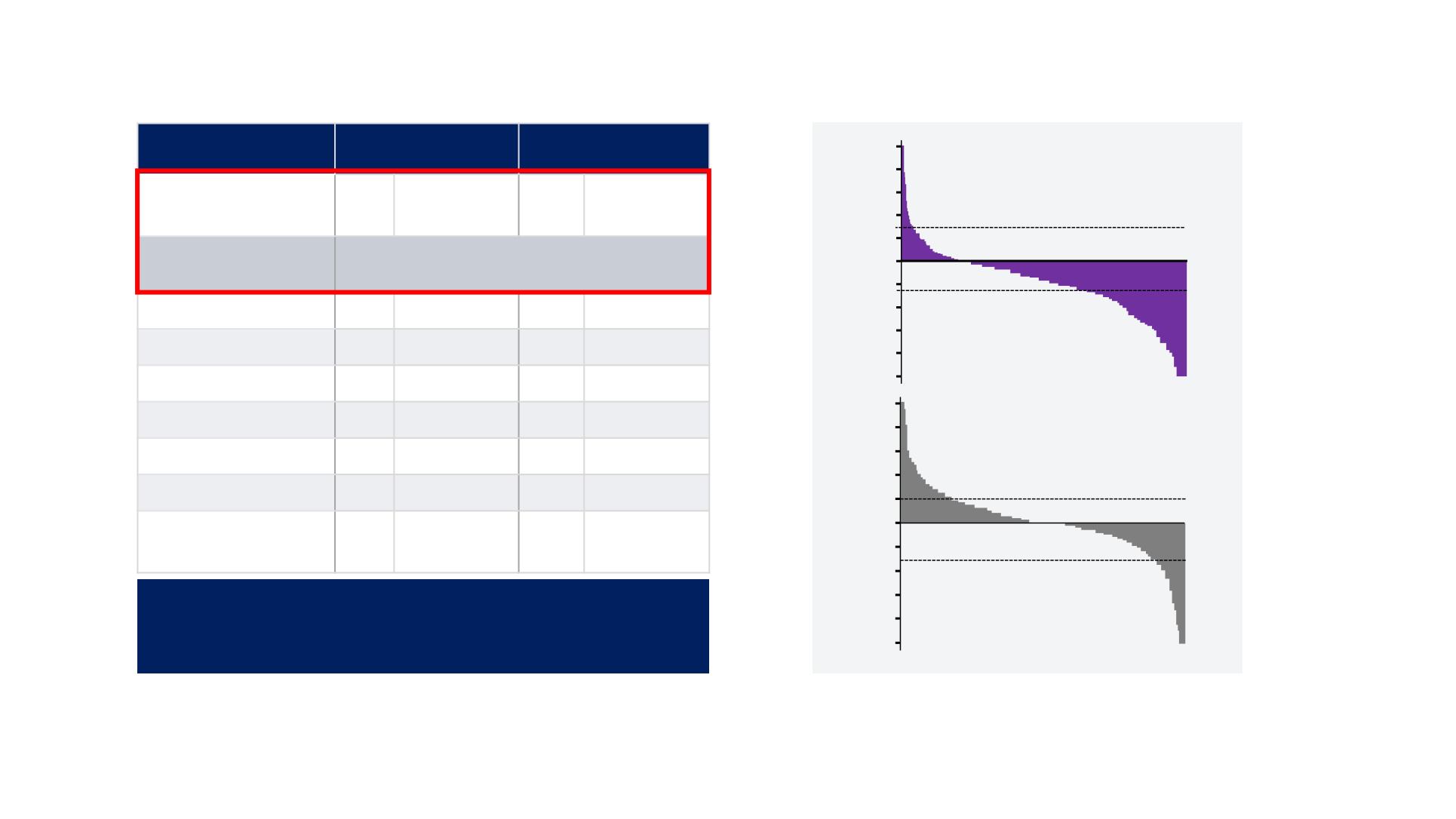
Maximum Change in Tumor Size by mRECIST
Maximum tumour shrinkage (%)
CR
(n=6)
Lenvatinib (N=433)
80
100
60
40
20
0
-20
-40
-60
-80
-100
Maximum tumour shrinkage (%)
80
100
60
40
20
0
-20
-40
-60
-80
-100
Sorafenib (N=436)
CR
(n=2)
CI = confidence interval; CR = complete response; DCR = disease control rate; NE = not evaluable; ORR = overall response rate; PD = progressive disease; PR =
partial response; SD = stable disease; wks = weeks.
Lenvatinib
(N=478)
Sorafenib
(N=476)
ORR, n, %
(95% CI)
115
24.1%
(20.2, 27.9)
44
9.2%
(6.6, 11.8)
Odds ratio
(95% CI), p value
3.13
(2.15, 4.56), p<0.00001
CR, n, %
6
1%
2
0.4%
PR, n, %
109
23%
42
8.8%
SD, n, %
246
52%
244
51.3%
SD ≥23 weeks, n, %
167
35%
139
29.2%
PD, n, %
71
15%
147
30.9%
Unknown/NE, n, %
46
10%
41
8.6%
DCR, n, %
(95% CI)
361
76%
(71.7, 79.4)
288
60.5%
(56.1, 64.9)
§
Percentage change in tumour size is truncated at 100%
(rectangles)
§
ORR is defined as CR + PR, according to mRECIST








