1. Kudo M
et al
.
Lancet
. 2018;pii:S0140-6736(18)30207–1.
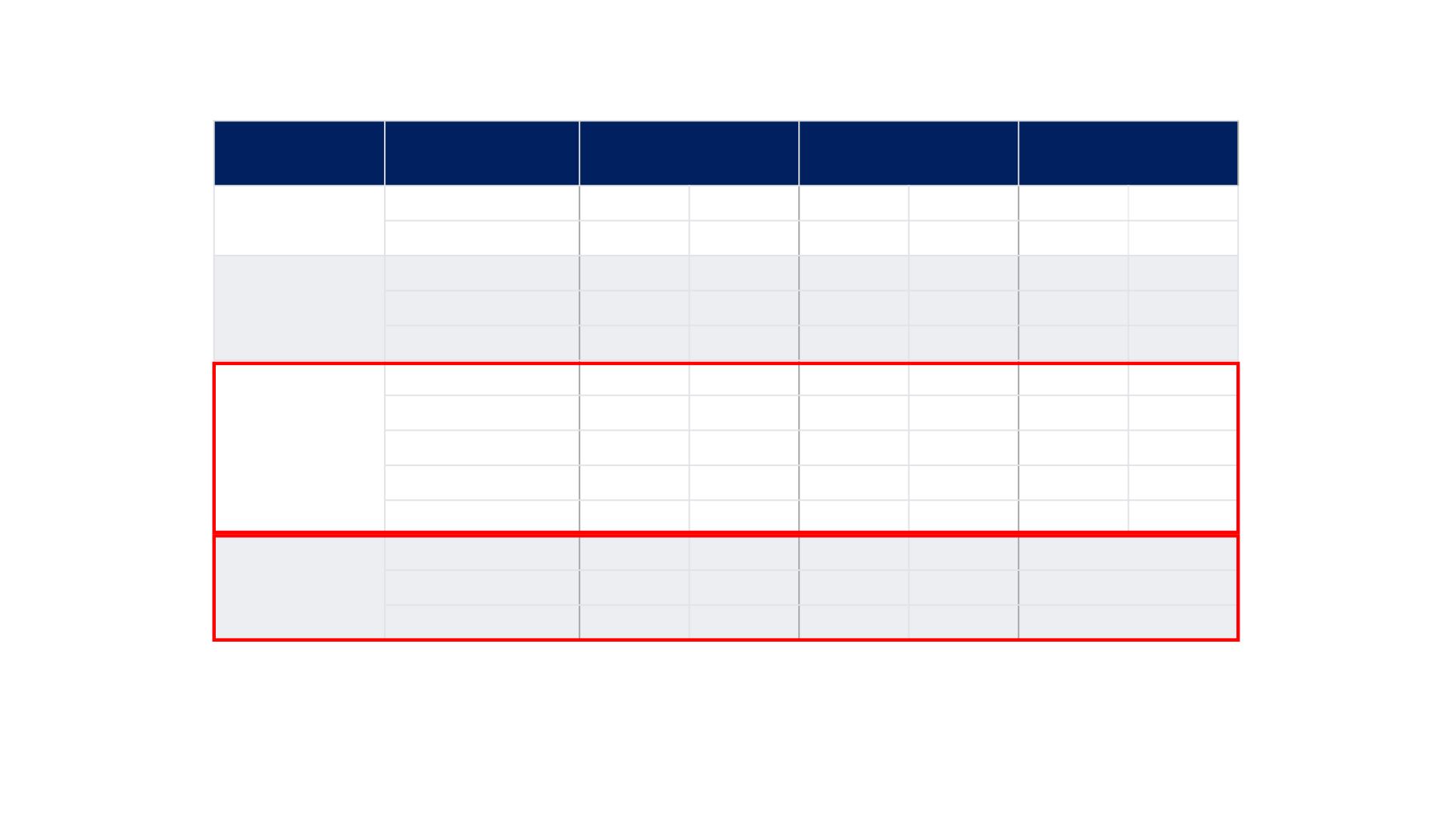
REFLECT TRIAL: Patient Characteristics
*One patient had no baseline target lesion.
AFP= alpha-fetoprotein; IQR = inter-quartile range; SD = standard deviation.
Characteristic
Category
Lenvatinib
(N=478)
Sorafenib
(N=476)
Total
(N=954)
Involved disease
sites
Liver
441
92%
430
90%
871
91%
Lung
163
34%
144
30%
307
32%
Involved disease
sites per
patient*, n, %
1
207
43%
207
43%
414
43%
2
167
35%
183
38%
350
37%
≥3
103
22%
86
18%
189
20%
Aetiology of
chronic liver
disease,
n, %
Hepatitis B
251
53%
228
48%
479
50%
Hepatitis C
91
19%
126
26%
217
23%
Alcohol
36
8%
21
4%
57
6%
Other
38
8%
32
7%
70
7%
Unknown
62
13%
69
14%
131
14%
Baseline AFP
concentration
(ng/mL)
Patients, n, %
471
99%
463
97%
934
98%
Mean, SD
17,507.5
105,137.4
16,678.5
94,789.5 17,096.5 100,088.8
Median, IQR
133.1
8.0–3,730.6
71.2
5.2–1,081.8 89.0 6.3–2,120.2








