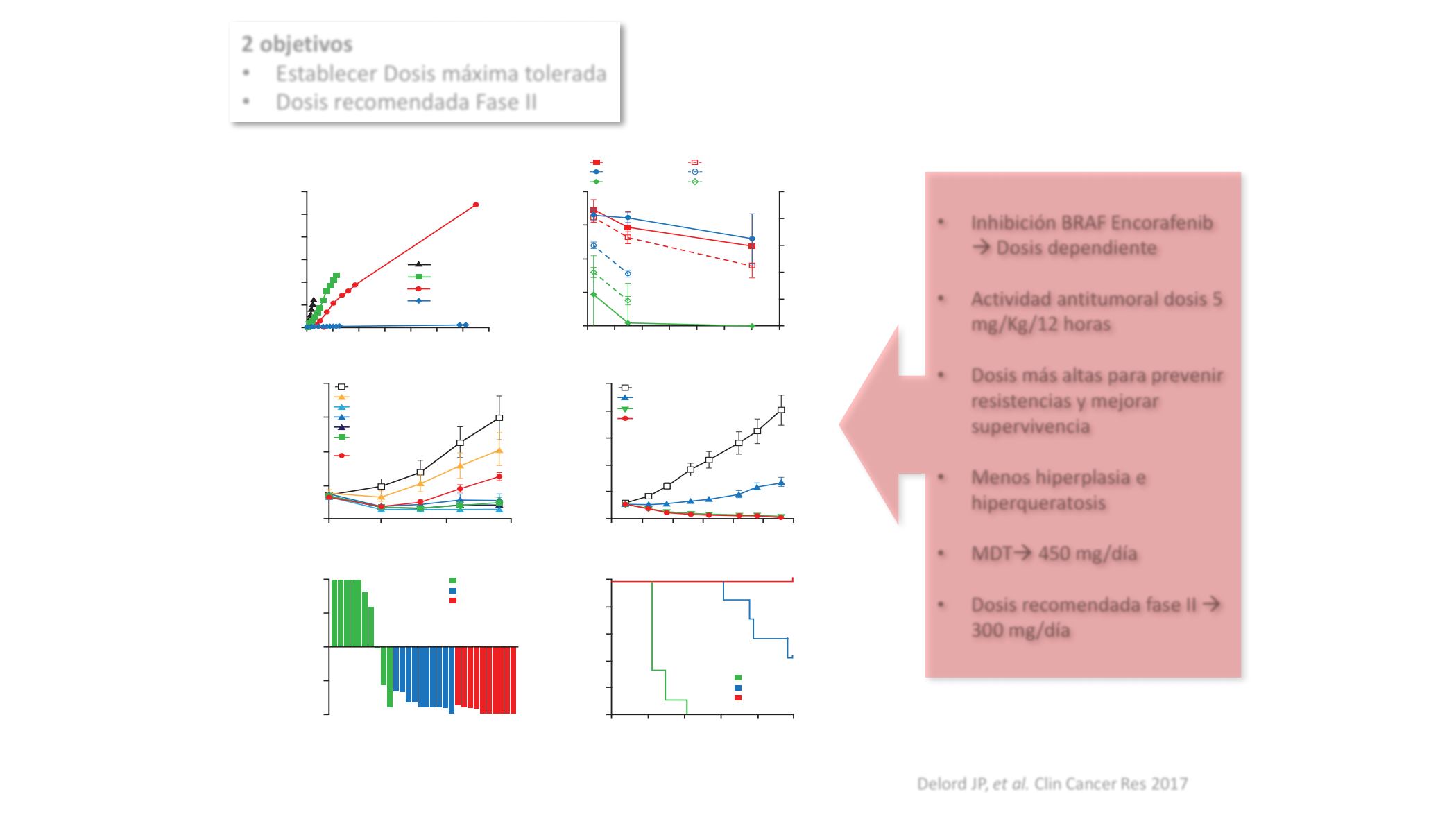
A
90
0
0 4 8 12 16
Time (hours)
pMEK RFU
20 24 28
Control
Vemurafenib
Dabrafenib
Encorafenib
15
30
45
60
75
C
2,000
0
15
Time postimplant (days)
Tumor volume (mm
3
)
mean ± SEM
20
25
0
500
1,000
1,500
B
100
1000.0
0
0.001
0.01
0.1
1
10
0 4 8 12 16
Time (hours)
pERK inhibition, %
(solid lines)
Estimated free drug
concentration,
µ
mol/L
(dashed lines)
20 24 28
60 mg/kg (pERK)
60 mg/kg (plasma)
6 mg/kg (pERK)
6 mg/kg (plasma)
0.6 mg/kg (pERK)
0.6 mg/kg (plasma)
25
50
75
Vehicle
0.6 mg/kg twice daily
6 mg/kg twice daily
60 mg/kg twice daily
300 mg/kg twice daily
Vemurafenib
60 mg/kg twice daily
Dabrafenib
100 mg/kg once
daily
D
2,500
20 25
Time postimplant (days)
Tumor volume (mm
3
)
mean ± SEM
45
40
35
50
0
500
1,000
2,000
1,500
Vehicle
1 mg/kg twice daily
5 mg/kg twice daily
20 mg/kg twice daily
30
E
100
Change in tumor volume
−
100
−
50
0
50
F
100
0 30
Time postimplant (days)
Conditional survival (%)
120
90
150
0
20
40
80
60
60
1 mg/kg twice daily
5 mg/kg twice daily
20 mg/kg twice daily
1 mg/kg twice daily
5 mg/kg twice daily
20 mg/kg twice daily
Figure 1.
Pharmacologic characterization of encorafenib.
A,
Impact of BRAF inhibitors on phosphorylated MEK (pMEK) in
BRAF
V600E
–
mutant cells.
B,
Tumor
pERK inhibition and plasma encorafenib concentration across varying doses (0.6
–
60 mg/kg) of encorafenib. Female nude mice bearing A375 (
BRAF
V600E)
human melanoma tumor xenografts were given a single oral dose of vehicle or encorafenib, and plasma and tumor samples were collected at
Delord et al.
Published OnlineFirst June 13, 2017; DOI: 10.1158/1078-0432.CCR-16-2923
2 objetivos
•
Establecer Dosis máxima tolerada
•
Dosis recomendada Fase II
•
Inhibición BRAF Encorafenib
à
Dosis dependiente
•
Actividad antitumoral dosis 5
mg/Kg/12 horas
•
Dosis más altas para prevenir
resistencias y mejorar
supervivencia
•
Menos hiperplasia e
hiperqueratosis
•
MDT
à
450 mg/día
•
Dosis recomendada fase II
à
300 mg/día
Delord JP,
et al.
Clin Cancer Res 2017








