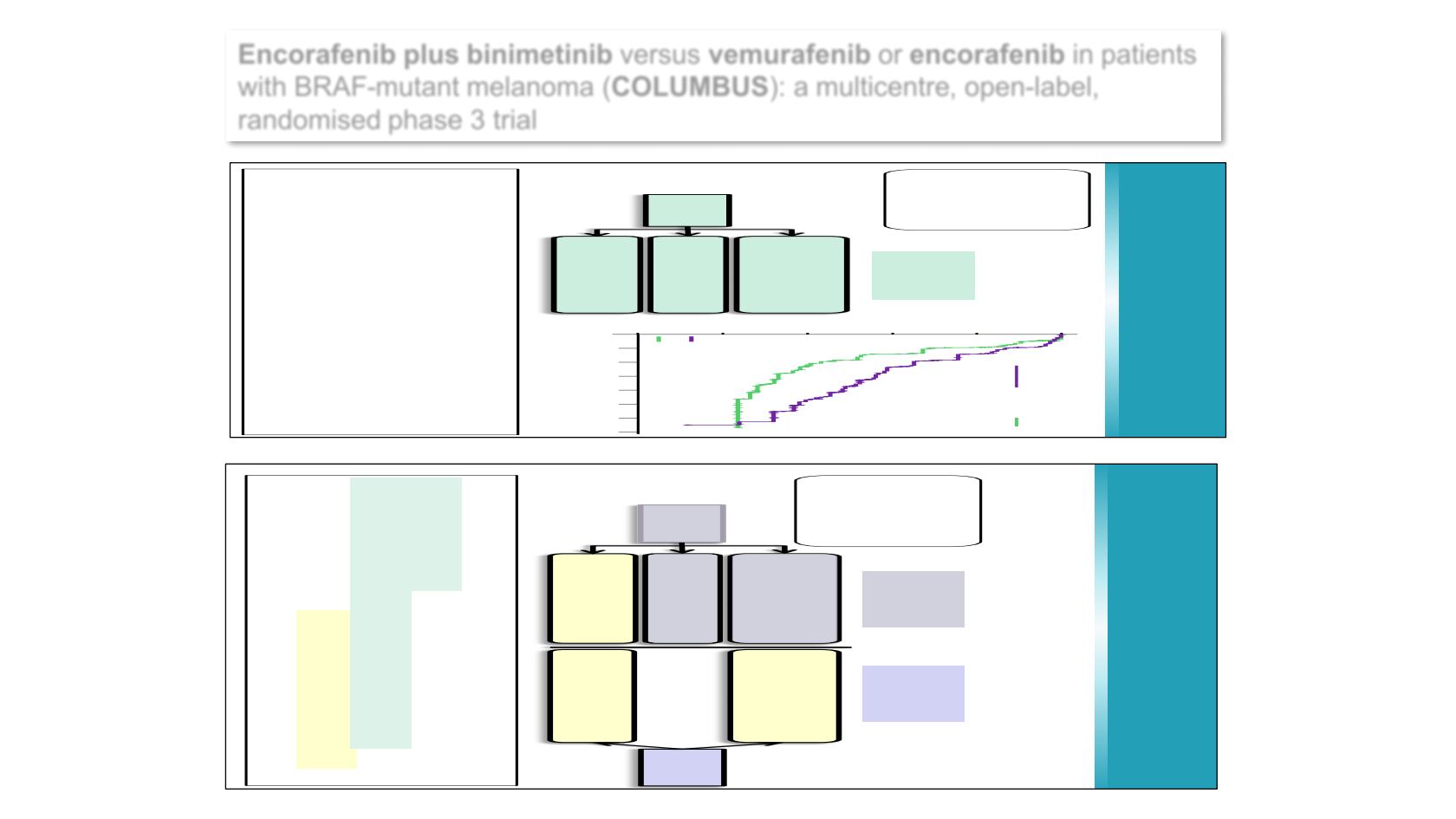
COLUMBUS Part 1
5
Untreatedorprogressed
on/afterprior first-line
immunotherapy
BRAFV600E
and/or
V600K
ECOGPS0–1
Randomized
1:1:1
ENCO450mgQD+BINI45mgBID
(COMBO450)
n=192
VEM960mgBID
n=191
ENCO300mgQD (ENCO300)
n=194
PART 1
N=577 randomized
*
PFS determinedbasedonblinded independent radiologyassessment
.
BINI=binimetinib;ECOGPS=EasternCooperativeOncologyGroup performance status;ORR=overall response rate;OS=overall survival;PFS=progression-free survival;
QoL=quality of life;VEM=vemurafenib.
Time (mo)
Progression-Free Survival (%)
100
0
4
8
12
16
20
24
28
0
20
40
60
80
COMBO450
VEM
MedianPFS inmonths (95%CI)
COMBO450
VEM
14.9
(11.0–18.5)
7.3
(5.6–8.2)
HR (95%CI), 0.54 (0.41–0.71)
P
<0.001
Dummer etal2016
Efficacyendpoints:
Primary:PFS*forCOMBO450vsVEM
Keysecondary (tested sequentially):
PFS*forCOMBO450vsENCO300 (Part 1:n=191)
Other secondary:
OS,ORR,QoL
Encorafenib plus binimetinib
versus
vemurafenib
or
encorafenib
in patients
with BRAF-mutant melanoma (
COLUMBUS
): a multicentre, open-label,
randomised phase 3 trial
Efficacy endpoints:
COLUMBUS Part 2
Randomized
1:1:1
ENCO450mgQD+BINI45mgBID
(COMBO450)
n=192
VEM960mgBID
n=191
ENCO300mgQD (ENCO300)
n=194
ENCO300mgQD+BINI45mgBID
(COMBO300)
n=258
ENCO300mgQD (ENCO300)
n=86
Randomized
3:1
PART 1
N=577 randomized
PART 2
N=344 randomized
PFS*for COMBO300vs ENCO300 (Part1+ Part 2:n=280)
*
PFS determinedbasedonblinded independent radiology assessment.
BINI=binimetinib;ECOGPS=EasternCooperativeOncologyGroup performance status;ORR=overall response rate;OS=overall survival; PFS=progression-free survival;
QoL=quality of life;VEM=vemurafenib.
Designed to isolate the contribution of BINI to combination therapy by maintaining the same dose of
ENCO in the combination (COMBO300) and comparator arms (ENCO300)
Untreatedorprogressed
on/afterprior first-line
immunotherapy
BRAFV600E
and/or
V600K
ECOGPS0–1
Primary: PFS* for COMBO450vs VEM
Keysecondary (tested sequentially):
PFS*for COMBO450vs ENCO300 (Part1: n=191)
Other secondary:
OS, ORR, QoL








