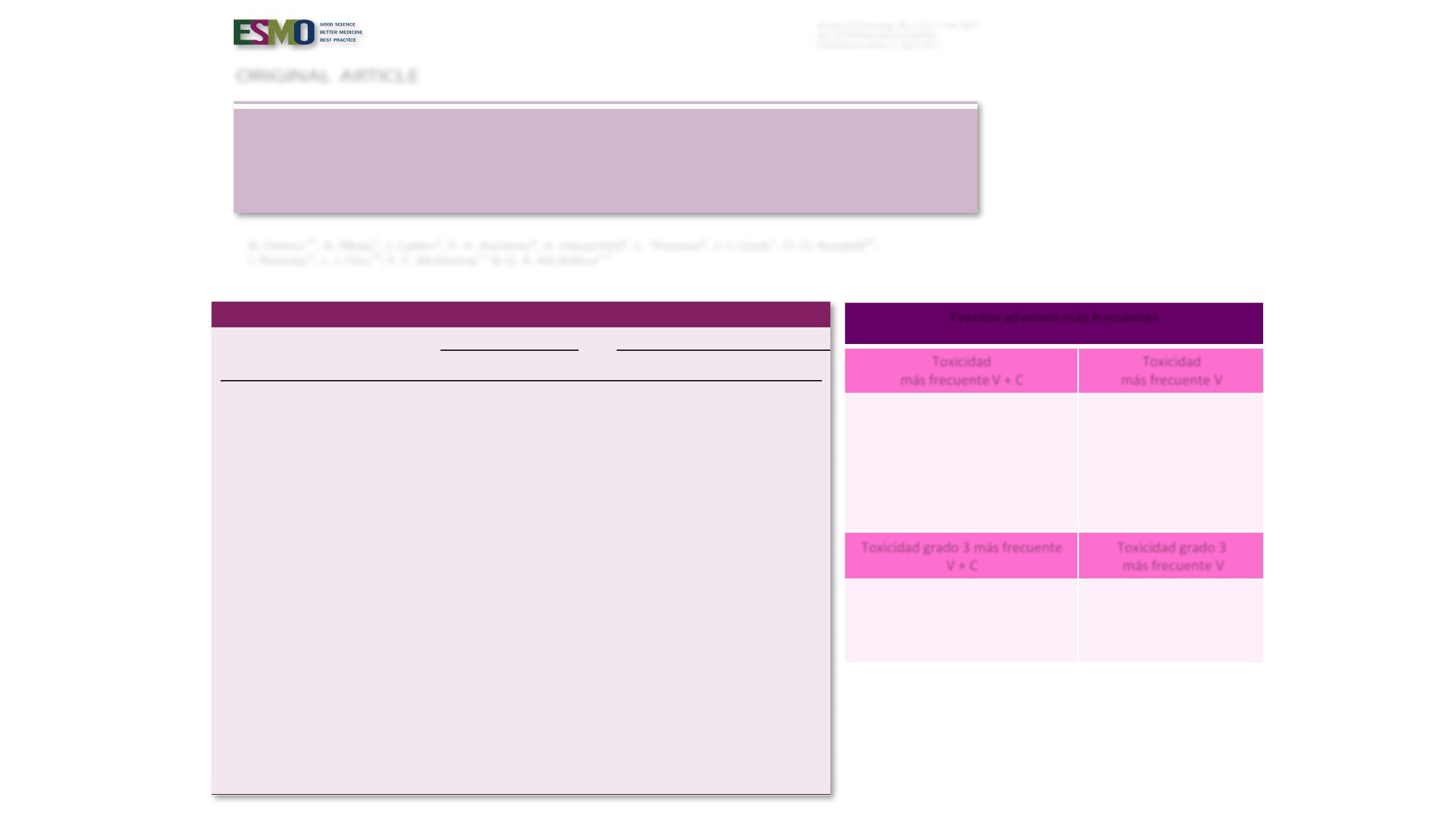
Table 1. Summary of AEs regardless of relationship to study drug (safety population
a
; data cut-off 30 September 2015)
Vemurafenib (
n
5
246)
Cobimetinib plus vemurafenib (
n
5
247)
A
ll grades Grade!
3
All grades
Grade
!
3
Any AE,
n
(%)
241 (98.0)
151 (61.4)
245 (99.2)
186 (75.3)
Most common AEs (
!
20% in either arm),
n
(%)
Rash
b
166 (67.5)
40 (16.3)
179 (72.5)
42 (17.0)
Arthralgia
103 (41.9)
12 (4.9)
94 (38.1)
6 (2.4)
Photosensitivity
c
93 (37.8)
0
118 (47.8)
11 (4.5)
Diarrhoea
82 (33.3)
2 (0.8)
150 (60.7)
16 (6.5)
Fatigue
82 (33.3)
7 (2.8)
91 (36.8)
11 (4.5)
Alopecia
75 (30.5)
1 (0.4)
41 (16.6)
1 (0.4)
Hyperkeratosis
67 (27.2)
6 (2.4)
25 (10.1)
1 (0.4)
Nausea
64 (26.0)
2 (0.8)
105 (42.5)
3 (1.2)
Pyrexia
59 (24.0)
0
71 (28.7)
3 (1.2)
Decreased appetite
50 (20.3)
1 (0.4)
50 (20.2)
0
Alanine aminotransferase level increase
44 (17.9)
15 (6.1)
65 (26.3)
28 (11.3)
c
-glutamyltransferase level increase
44 (17.9)
25 (10.2)
54 (21.9)
36 (14.6)
Vomiting
34 (13.8)
2 (0.8)
63 (25.5)
4 (1.6)
Aspartate aminotransferase level increase
31 (12.6)
5 (2.0)
60 (24.3)
22 (8.9)
Serous retinopathy
d
9 (3.7)
0
67 (27.1)
7 (2.8)
Blood creatine phosphokinase level increase
7 (2.8)
1 (0.4)
87 (35.2)
30 (12.1)
Other selected AEs,
n
(%)
cuSCC
31 (12.6)
31 (12.6)
10 (4.0)
9 (3.6)
Keratoacanthoma
23 (9.3)
21 (8.5)
4 (1.6)
3 (1.2)
Decreased ejection fraction
13 (5.3)
3 (1.2)
29 (11.7)
5 (2.0)
QT prolongation
13 (5.3)
3 (1.2)
11 (4.5)
3 (1.2)
Adapted from
The Lancet
, Ascierto PA et al., “Overall survival with cobimetinib combined with vemurafenib in advanced
BRAF
V600
-mutated melanoma:
Annals of Oncology
Original article
Eventos adversos más frecuentes
Toxicidad
más frecuente V + C
Toxicidad
más frecuente V
Elevación CPK (32%)
Diarrea (27.4%)
Retinopatía (23.4%)
Náuseas (16.5%)
Elevación GPT (11.7%)
Vómitos (11.7%)
Fotosensibilidad (10%)
Hiperqueratosis (17%)
Alopecia (1%)
Toxicidad grado 3 más frecuente
V + C
Toxicidad grado 3
más frecuente V
Elevación GOT (6.9%)
Diarrea (5.7%)
Elevación GPT (5.2%)
Náuseas (16.5%)
Carcinomas epidermoides
(9%)
Quératoacantomas (7.3%)
ORIGINAL ARTICLE
Incidence, course, and management of toxicities
associated with cobimetinib in combination with
vemurafenib in the coBRIM study
B. Dre´no
1
* , A. Ribas
2
, J. Larkin
3
, P. A. Ascierto
4
, A. Hauschild
5
, L. Thomas
6
, J.-J. Grob
7
, D. O. Koralek
8†
,
I. Rooney
9
, J. J. Hsu
10
, E. F. McKenna
11
& G. A. McArthur
12
1
Department f Dermato Cancerology, Nantes University, Nantes, France;
2
Department of Medicine, Hematology/Oncology, Jonsson Comprehensive Cancer
Center, The University of California, Los Angeles, Los Angeles, USA;
3
Department of Medicine, Royal Marsden NHS Foundation Trust, London, UK;
4
Unit of
Melanoma, Cancer Immunotherapy and Innovative Therapy, Istituto Nazionale Tumori Fondazione G. Pascale, Naples, Italy;
5
Department of Dermatology, University
Hospital Schleswig-Holstein, Kiel, Germany;
6
Department of Service de Dermatology, Centre Hospitalier Lyon Sud, Pierre-Be´nite;
7
Dermatology and Cutaneous
Oncology, Aix-Marseille University Hoˆpital de la Timone AP-HM, Marseille, France;
8
Departments of Clinical Development;
9
PDCO;
10
Biostatistics;
11
Medical Affairs,
Genentech, Inc., South San Francisco, USA;
12
Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, Australia
*
Correspondence to:
Dr Brigitte Dre´no, Department of Dermato Cancerology, Nantes University, University Hospital, CHU Nantes – Place Alexis Ricordeau, 44093 Nantes
Cedex 01, France. Tel:
þ
33-2-40-08-31-18; E-mail:
brigitte.dreno@wanadoo.fr†
Former employee of Genentech, Inc.
Background:
In the coBRIM phase III trial, the addition of cobimetinib, an MEK inhibitor, to vemurafenib, a BRAF inhibitor,
significantly improved progression-free survival [hazard ratio (HR), 0.58;
P
<
0.0001] and overall survival (HR, 0.70;
P
¼
0.005) in
advanced
BRAF
-mutated melanoma. Here, we report on the incidence, course, and management of key adverse events (AEs) in
the coBRIM study.
Patients and methods:
Patients were randomly assigned 1:1 to receive vemurafenib (960 mg twice a day) and either
cobimetinib (60 mg once a day, 21 days on/7 days off) or placebo. In addition to standard safety evaluations, patients
underwent regular ophthalmic, cardiac, and dermatologic surveillance examinations.
Results:
Of 495 patients recruited to the study, 493 patients received treatment and constituted the safety population
(cobimetinib combined with vemurafenib, 247; vemurafenib, 246). At data cut-off (30 September 2015), median follow-up was
18.5 months. Nearly every patient experienced an AE. In patients who received cobimetinib combined with vemurafenib, the
frequency of grade
#
3 AEs was higher than in patients who received vemurafenib alone (75% versus 61%). Most AEs, including
grade
#
3 AEs, occurred within the first treatment cycle. After the first cycle (28 days), the incidence of common AEs (rash, diar-
rhoea, photosensitivity, elevated creatine phosphokinase, serous retinopathy, pyrexia, and liver laboratory abnormalities)
decreas d substantially over time. Most AEs were managed conservatively by supportive care measures, dose modifications of
study treatment, and, occasionally, permanent treatment discontinuation.
Conclusions:
These data indicate that most AEs arising from treatment with cobimetinib combined with vemurafenib
generally occur early in the treatment course, are mild or moderate and are manageable by patient monitoring, dose
modification and supportive care.
ClinicalTrials.gov:
NCT01689519.
Key words
:
cobimetinib, MEK inhibition, melanoma, safety
Introduction
Targeted therapies have revolutionized the management of advanced
BRAF
-mutated melanoma, specifically BRAF inhibitors (BRAFi)
such as vemurafenib and dabrafenib and MEK inhibitors (MEKi)
such as cobimetinib and trametinib. Combination regimens of
BRAFi and MEKi have improved clinical benefit compared with
V
C
The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
All rights reserved. For Permissions, please email:
journals.permissions@oup.com.
Annals of Oncology
28: 1137–1144, 2017
doi:10.1093/annonc/mdx040
Published online 21 April 2017








