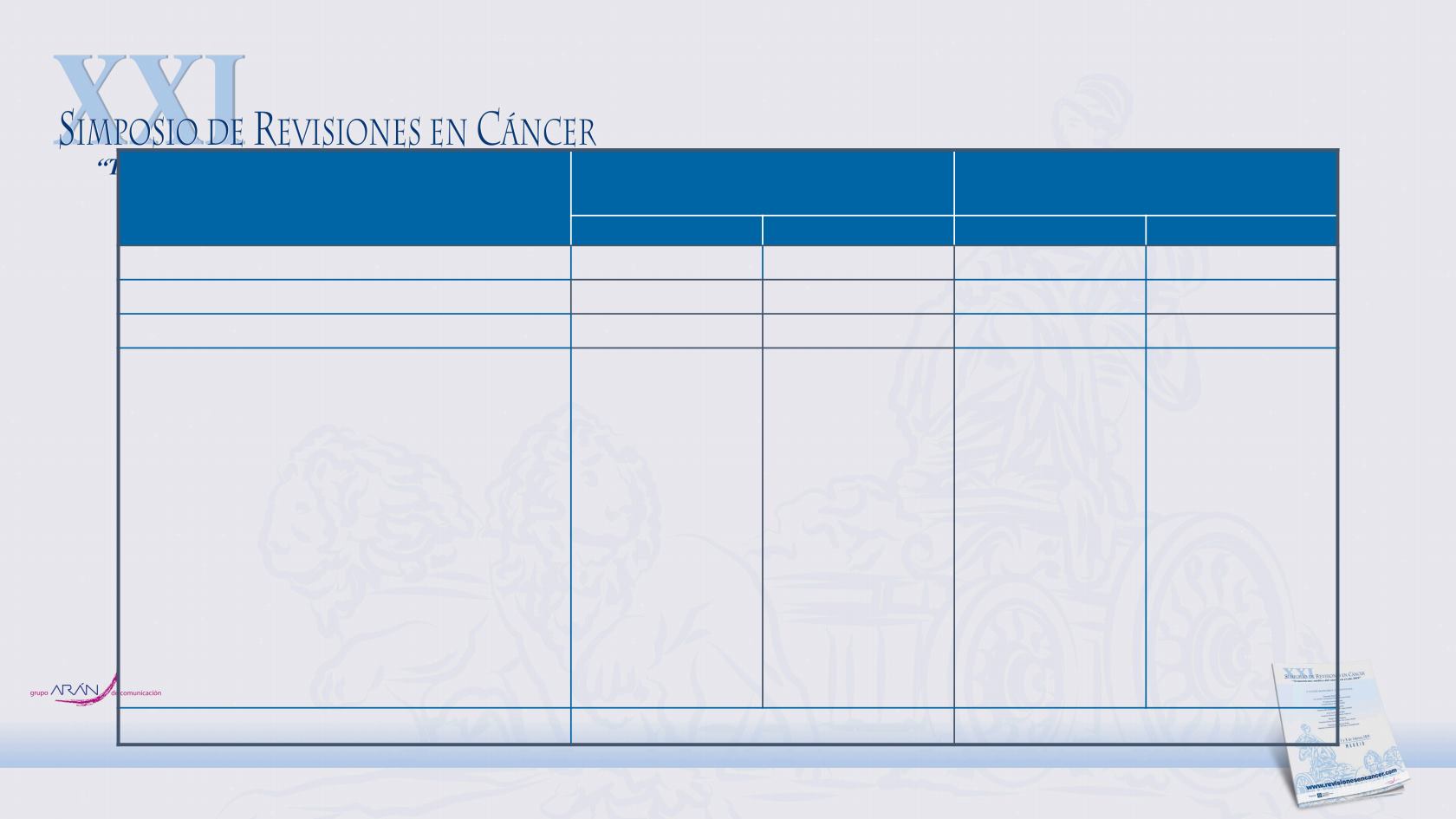
Treatment-Related AEs
a
Includes events reported between first dose and 30 days after last dose of study drug.
b
In either group.
c
Treatment-related deaths in the nivolumab group were neurologic neoplastic syndrome (n = 1) and atrial
fibrillation with acute heart failure (n = 1); deaths in the chemotherapy group included febrile neutropenia and sepsis (n = 2) and pancytopenia (n = 1)
TRAE,
a
n (%)
Nivolumab
(n = 282)
Chemotherapy
(n = 265)
Any grade
Grade 3–4
Any grade
Grade 3–4
Any TRAE
156 (55)
39 (14)
239 (90)
194 (73)
Serious TRAEs
37 (13)
22 (8)
87 (33)
81 (31)
TRAE leading to discontinuation
17 (6)
12 (4)
38 (14)
25 (9)
Most frequent TRAEs (≥15%
b
)
Asthenia
Fatigue
Decreased appetite
Anemia
Nausea
Platelet count decreased
Thrombocytopenia
White blood cell count decreased
Leukopenia
Neutropenia
Neutrophil count decreased
25 (9)
25 (9)
21 (7)
13 (5)
14 (5)
5 (2)
5 (2)
4 (1)
4 (1)
4 (1)
0
2 (1)
0
1 (0.4)
0
0
1 (0.4)
0
1 (0.4)
0
1 (0.4)
0
42 (16)
54 (20)
40 (15)
147 (56)
47 (18)
63 (24)
80 (30)
45 (17)
43 (16)
91 (34)
58 (22)
17 (6)
13 (5)
5 (2)
68 (26)
2 (1)
34 (13)
56 (21)
30 (11)
31 (12)
73 (28)
45 (17)
Treatment-related deaths
c
2 (1)
3 (1)
36








