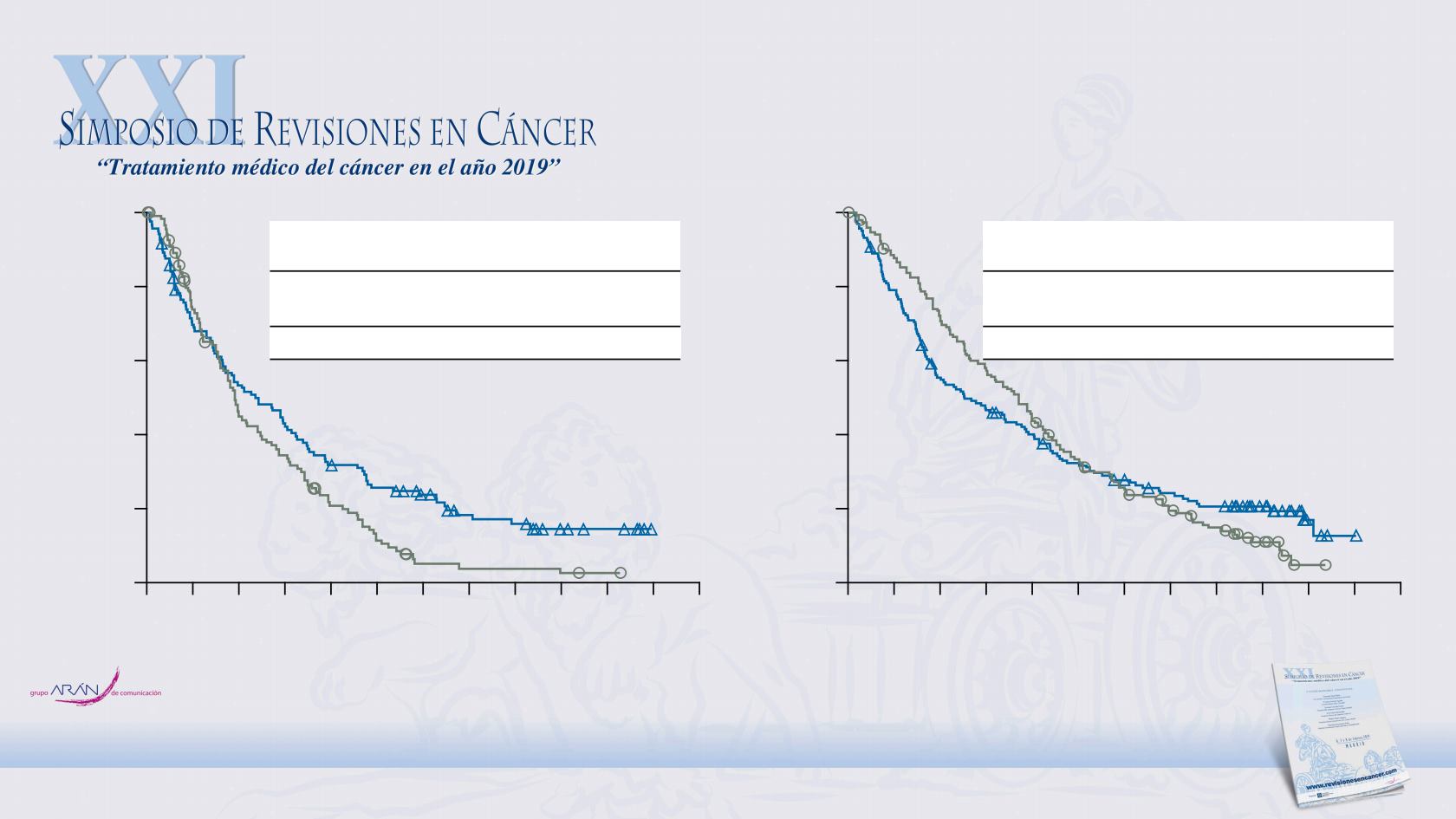
OS by Response to Prior Platinum Therapy
a
Minimum follow-up: 15.8 months;
59 patients (21%) in the nivolumab arm and 40 patients (14%) in the chemotherapy arm were censored.
a
Platinum sensitivity was defined by a cutoff of 90 days
progression-free interval after completion of platinum therapy
34
Nivolumab
(n = 121)
Chemotherapy
(n = 125)
Median OS
7.0
5.7
(95% CI), mo
(4.9–9.4)
(4.7–7.3)
HR (95% CI)
0.71 (0.54–0.94)
0 3 6 9 12 15 18 21 24 27 30 33 36
0
40
60
80
100
OS (%)
Months
20
Platinum resistant
0 3 6 9 12 15 18 21 24 27 30 33 36
0
40
60
80
100
OS (%)
Months
20
Platinum sensitive
Nivolumab
Chemotherapy
Nivolumab
Chemotherapy
No. at risk
Nivolumab
Chemo
121
125
81
85
62
51
49
39
37
22
29
12
23
4
15
3
13
3
7
2
5
1
0
0
0
0
No. at risk
Nivolumab
Chemo
163
160
128
136
88
110
74
89
62
68
49
50
40
37
34
27
29
19
19
9
4
1
1
0
0
0
Nivolumab
(n = 163)
Chemotherapy
(n = 160)
Median OS
7.6
11.1
(95% CI), mo
(5.6–11.2)
(8.9–12.6)
HR (95% CI)
0.98 (0.77–1.25)








