Pujol J, et al. Ann Oncol 2018;29(suppl 5):Abstr 1664O
Primary endpoint
•
ORR at 6 weeks (investigator assessed)
R
2:1
PD
PD
Stratification
•
Sensitive vs. refractory disease
•
PS (0–1 vs. 2)
•
Limited vs. extensive disease
•
Gender
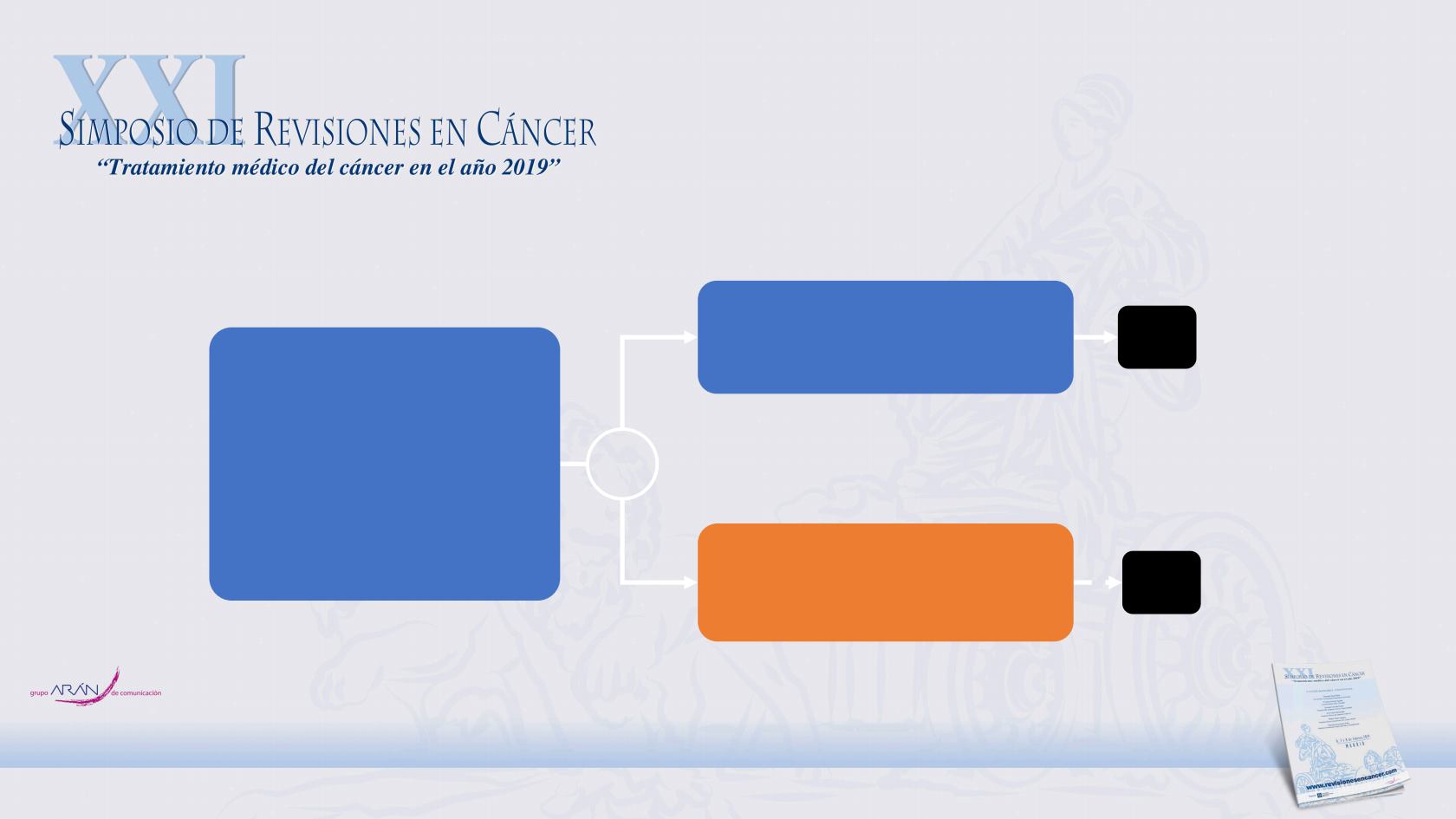
Key patient inclusion criteria
•
ED or LD SCLC
•
Progressive disease after 1L
chemotherapy
•
No brain metastases
•
PS 0–2
(n=73)
Chemotherapy:
topotecan (oral or IV) q3w or
carboplatin-etoposide q3w*
(n=24)
Atezolizumab 1200 mg q3w
(n=49)
*Maximum 6 cycles
IFCT-1603 Trial: Randomized phase II study of atezolizumab or chemotherapy
as second-line therapy








