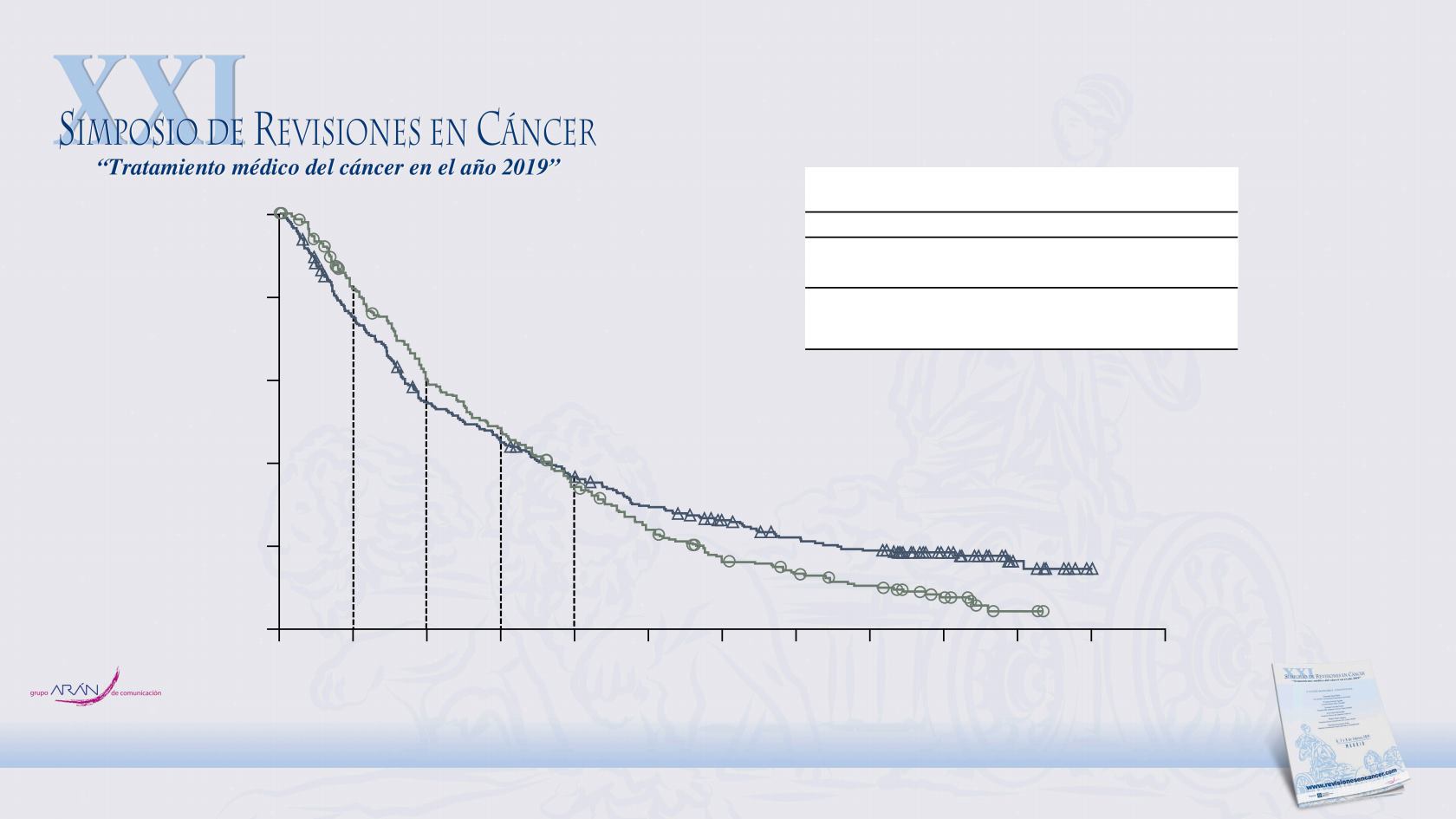
Nivolumab
(n = 284)
Chemotherapy
(n = 285)
No. of events
225
245
Median OS
7.5
8.4
(95% CI), mo
(5.6–9.2)
(7.0–10.0)
HR (95% CI)
P
value
0.86 (0.72–1.04)
0.11
Minimum follow-up: 15.8 months; 59 patients (21%) in the nivolumab arm and 40 patients (14%) in the chemotherapy arm were censored
100
0 3 6 9 12 15 18 21 24 27 30 33 36
0
40
60
80
OS (%)
Months
20
63
41
284
285
209
221
150
161
123
128
99
90
78
62
49
30
42
22
26
11
9
2
1
0
0
0
12-mo OS = 37%
12-mo OS = 34%
6-mo OS = 54%
6-mo OS = 60%
Nivolumab
Chemotherapy
31
No. at risk
Nivolumab
Chemo
1.46
1.02–2.11
1.03
0.72–1.49
0.83
0.50–1.38
0.59
0.35–1.00
0.49
0.33–0.72
Exploratory Piecewise HR
(95% CI)








