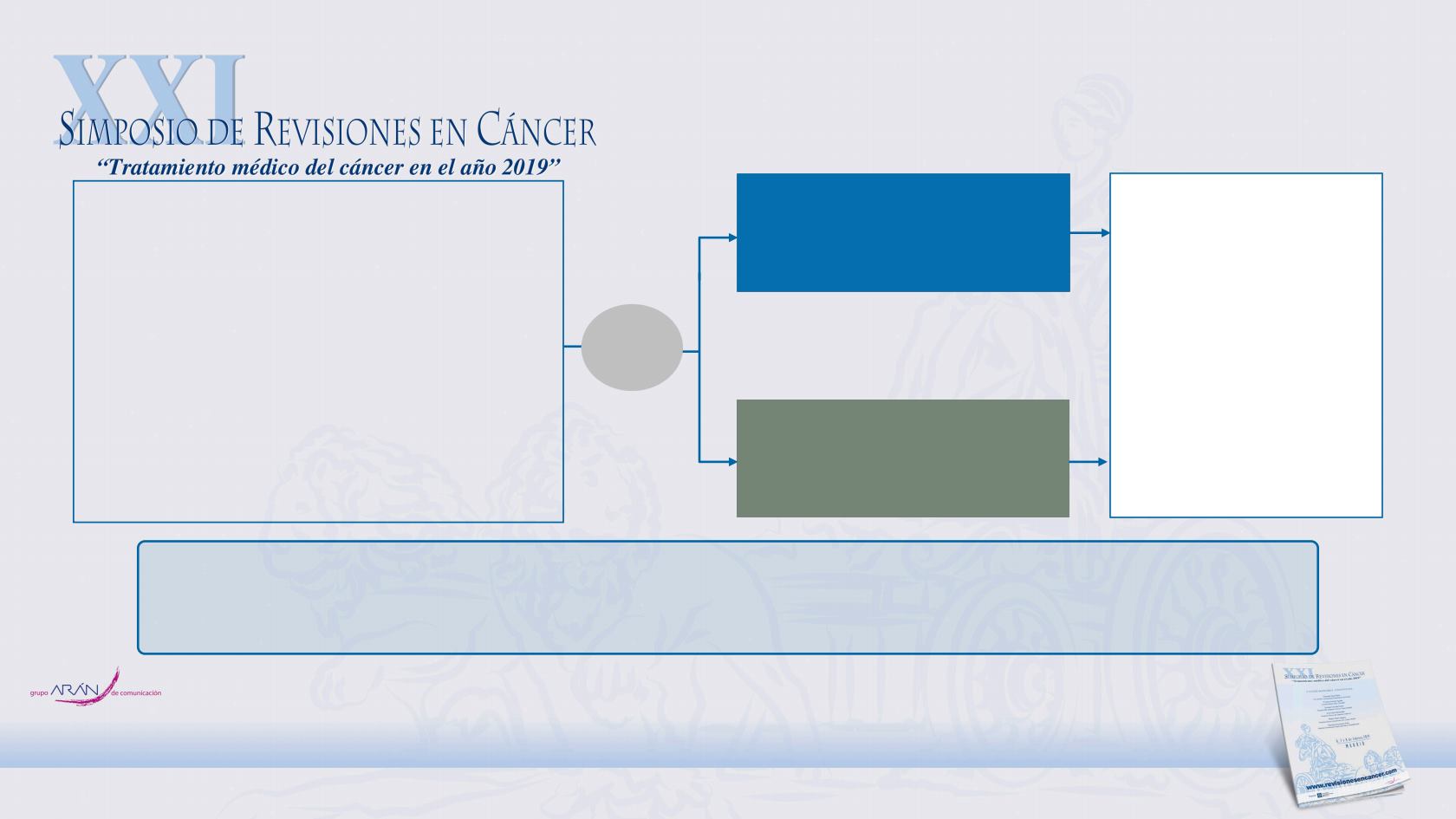
CheckMate 331 Study Design
30
Primary endpoint: OS
Secondary endpoints: PFS
g
and ORR
g
(investigator assessed)
Nivolumab
240 mg Q2W
n = 284
Chemotherapy:
topotecan (IV or oral)
d
or
amrubicin IV
e,f
n = 285
Treat until disease
progression
g,h
or
unacceptable toxicity
•
Database lock: 28 September 2018; minimum follow-up for OS: 15.8 months
•
Median follow-up
i
: 7.0 months (nivolumab), 7.6 months (chemotherapy)
Key eligibility criteria
•
LD- or ED-SCLC at diagnosis
•
Relapse after platinum-based 1L
chemotherapy
a
•
ECOG PS 0–1
Stratification factors
•
Response to prior platinum-based
treatment (sensitive vs resistant
b
)
•
Brain metastases at baseline
(yes vs no)
R
c
1:1
Reck M. ESMO IO December 18








