Kudo M
et al
.
Lancet
. 2018;pii:S0140-6736(18)30207–1.
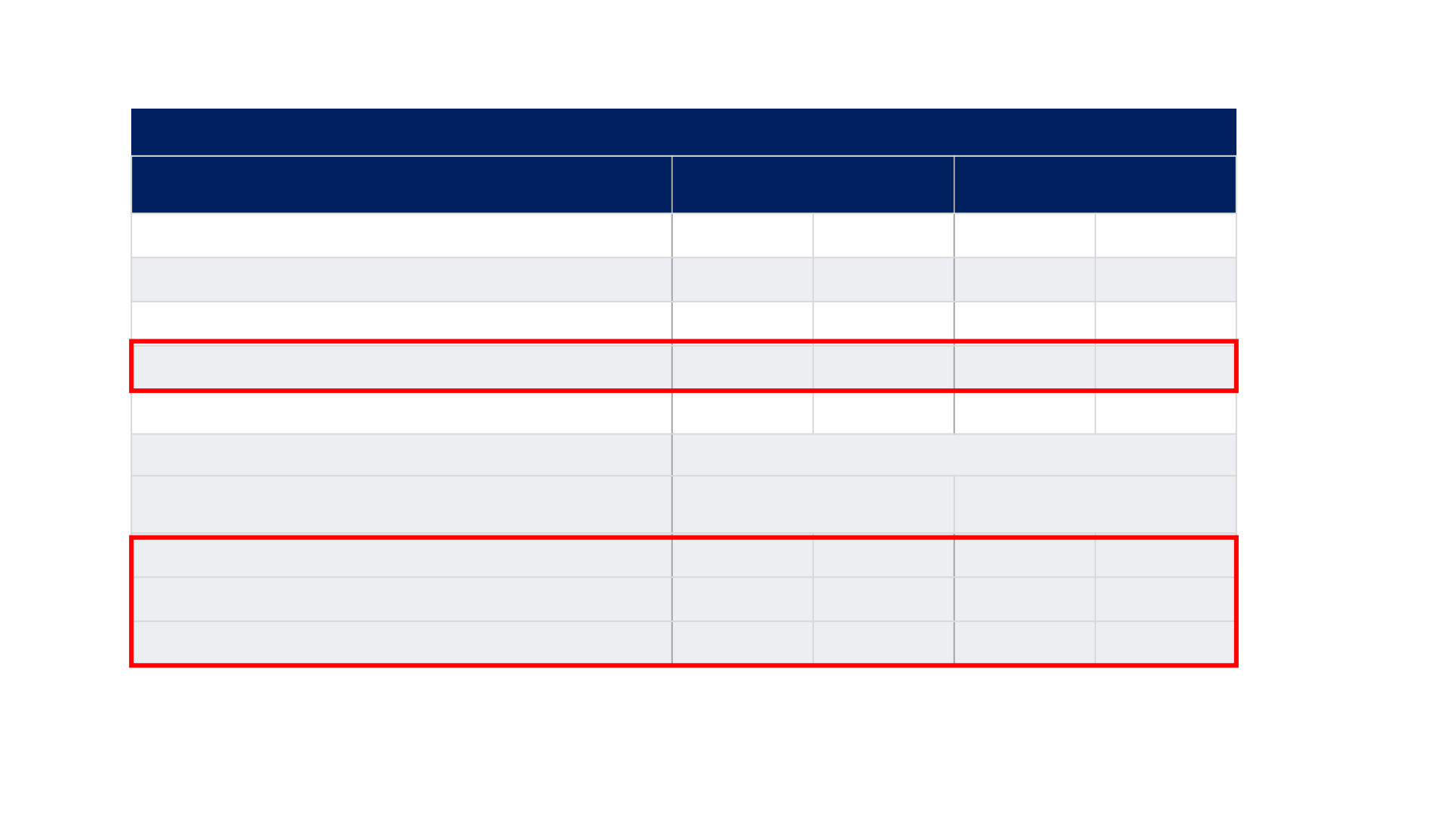
REFLECT TRIAL: Overview of TEAEs and dose modification
TEAEs
n, %
Lenvatinib
(N=476)
Sorafenib
(N=475)
Any TEAEs
470
99%
472
99%
Treatment-related TEAEs
447
94%
452
95%
Any TEAEs of grade ≥3
357
75%
316
67%
Treatment-related TEAEs of grade ≥ 3
270
57%
231
49%
Any serious AEs
205
43%
144
30%
Dose modifications
n, %
Lenvatinib
(N=476)
Sorafenib
(N=475)
Drug interruption due to treatment-related TEAEs
190
40%
153
32%
Dose reduction due to treatment-related TEAEs
176
37%
181
38%
Drug withdrawal due to treatment-related TEAEs
42
9%
34
7%
AE = adverse events; TEAE = treatment-emergent adverse event.








