K
EY
P
OINTS
The REFLECT trial demonstrates:
•
No notable differences in AE’s between the different weight based starting doses
•
Comparable efficacy results between the different weight based starting doses
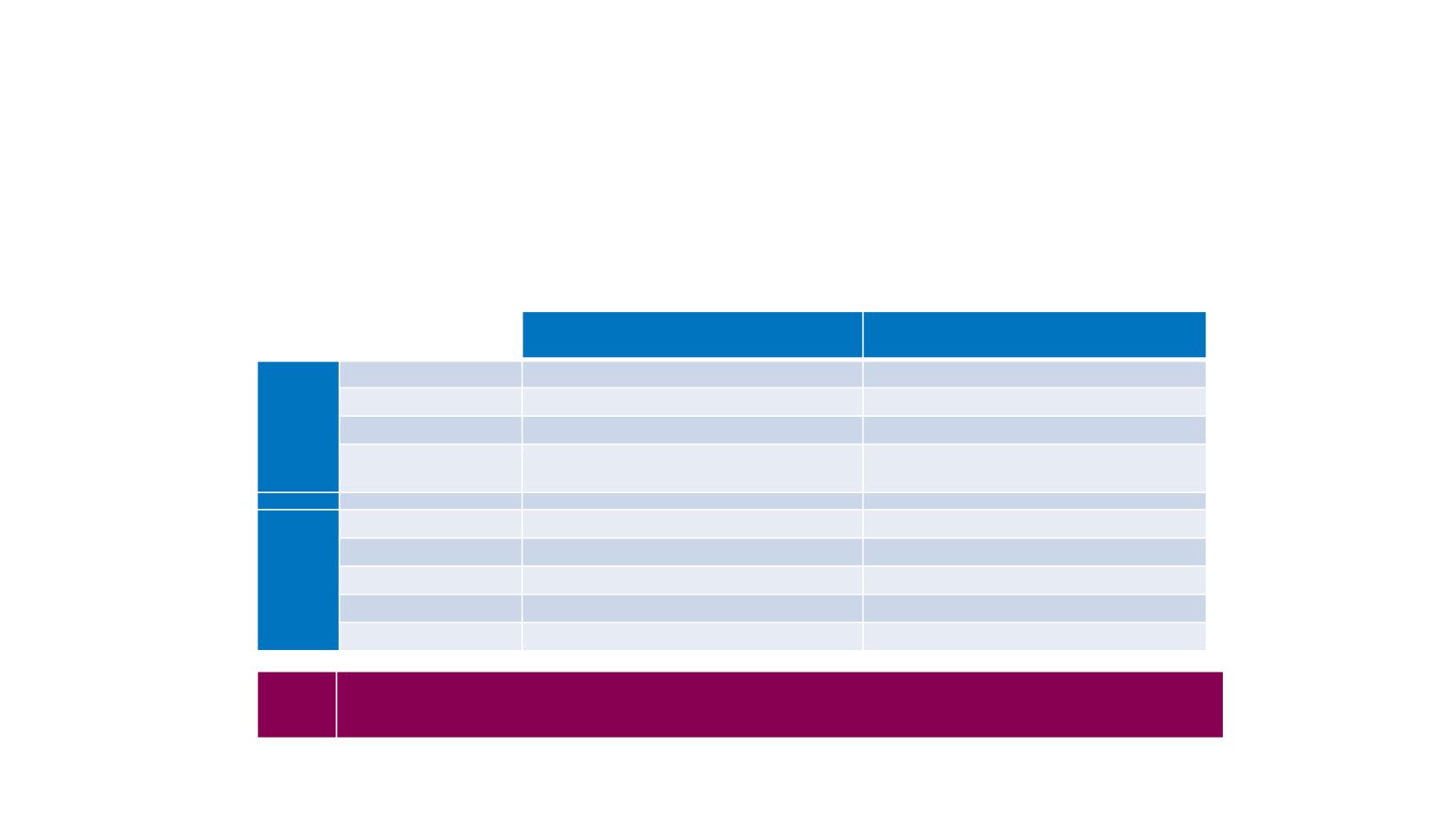
Safety and efficacy of lenvatinib by starting dose based on bodyweight
in patients (pts) with unresectable hepatocellular carcinoma (uHCC) in
REFLECT
•
In REFLECT, a multicentre, open-label, non-inferiority trial, pts with uHCC were
randomized 1:1 to:
•
lenvatinib (BW <60 kg: 8mg/d; BW ≥60 kg: 12 mg/d; QD)
•
sorafenib (400 mg BID)
lenvatinib starting dose
BW <60 kg: 8mg/d; QD (n=151)
lenvatinib starting dose
BW ≥60 kg: 12 mg/d; QD (n=327)
Efficacy
metrics
Median OS
13.4 months
13.7 months
ORR
22.2%
24.9%
Median PFS
7.4 months
7.4 months
Median duration of
treatment
5.6 months
6.3 months
Most common
any-grade
adverse events
Hypertension
43%
42%
Diarrhoea
35%
40%
Decreased appetite
33%
35%
Weight loss
29%
32%
Fatigue
28%
31%
1. Okusaka T et al. Presented at ASCO GI 2019








