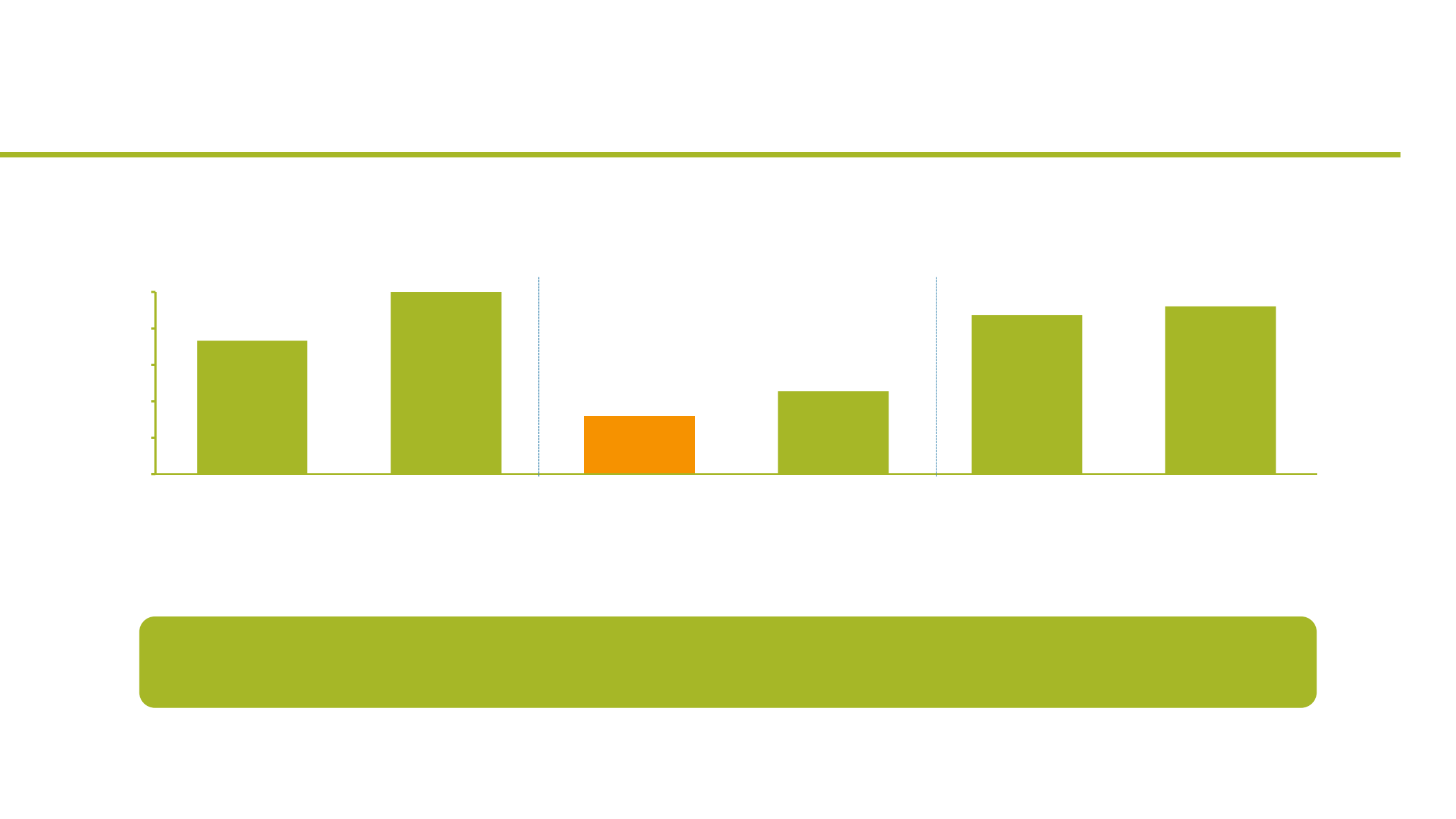
•
In total, of 111 patients treated by BRAFi or/and MEKi, 212 related or possibly related cutaneous AEs
were identified
Less proliferative keratinocytic cutaneous adverse events
Goldinger SM, et al. Journal of Clinical Oncology 35, no. 15_suppl (May 20 2017) 9590-9590
Patients with at least one cutaneous AE (%)
0
20
40
60
80
100
Encorafenib
(n=26)
Vemurafenib
(n=6)
Encorafenib + Binimetinib
(n=48)
Dabrafenib + Trametinib
(n=11)
Trametinib
(n=8)
Binimetinib
(n=25)
Capacity to inhibit the dimer can explain the low paradoxical ERK activation
observed in healthy cells and the
Study design: retrospective analysis, one center analysis (University Hospital of Zurich)
AE, adverse event; BRAFi, BRAF inhibitor; MEKi, MEK inhibitor.








