100% (IQR 93–100) of planne
99·6% (80–100) of planned do
dose intensity was 86% (55–1
encorafenib and 94% (74–1
distribution of dose intensity
shown in the appendix (p 25)
the encorafenib plus binim
achieve a dose intensity of
encorafenib and 144 [75%]
patients receiving the combi
50% dose intensity (five [
11 [6%] for binimetinib).
192 patients in the encorafe
186 patients in the vemurafe
intensity of 80–100%, and 4
group and 13 (7%) in the ve
dose intensity of less than 50
192 patients were assessa
encorafenib plus binimetinib
186 patients in the vemurafeni
events occurring in at least 10
4 adverse events occurring in
treatment group are summari
[p 13] lists all grade 3–4 adve
groups; as per the study prot
and therefore were not incl
adverse events reportedmore f
plus binimetinib group th
vemurafenib groups (with a
patients of 10% or higher)
effects (diarrhoea, constipatio
pain), predominantly asympt
phosphokinase, and blurred
events reported at a lower fre
proportion of patients of 10%
plus binimetinib group th
vemurafenib groups were
(eg, pruritus, hyperkeratosis, r
plantar keratoderma, palmo
syndrome, dry skin, skin pa
and sunburn), alopecia,
arthralgia, myalgia, pain in
appetite, musculoskeletal pain
Grade 3–4 adverse events we
in the encorafenib plus binime
than in either the encorafe
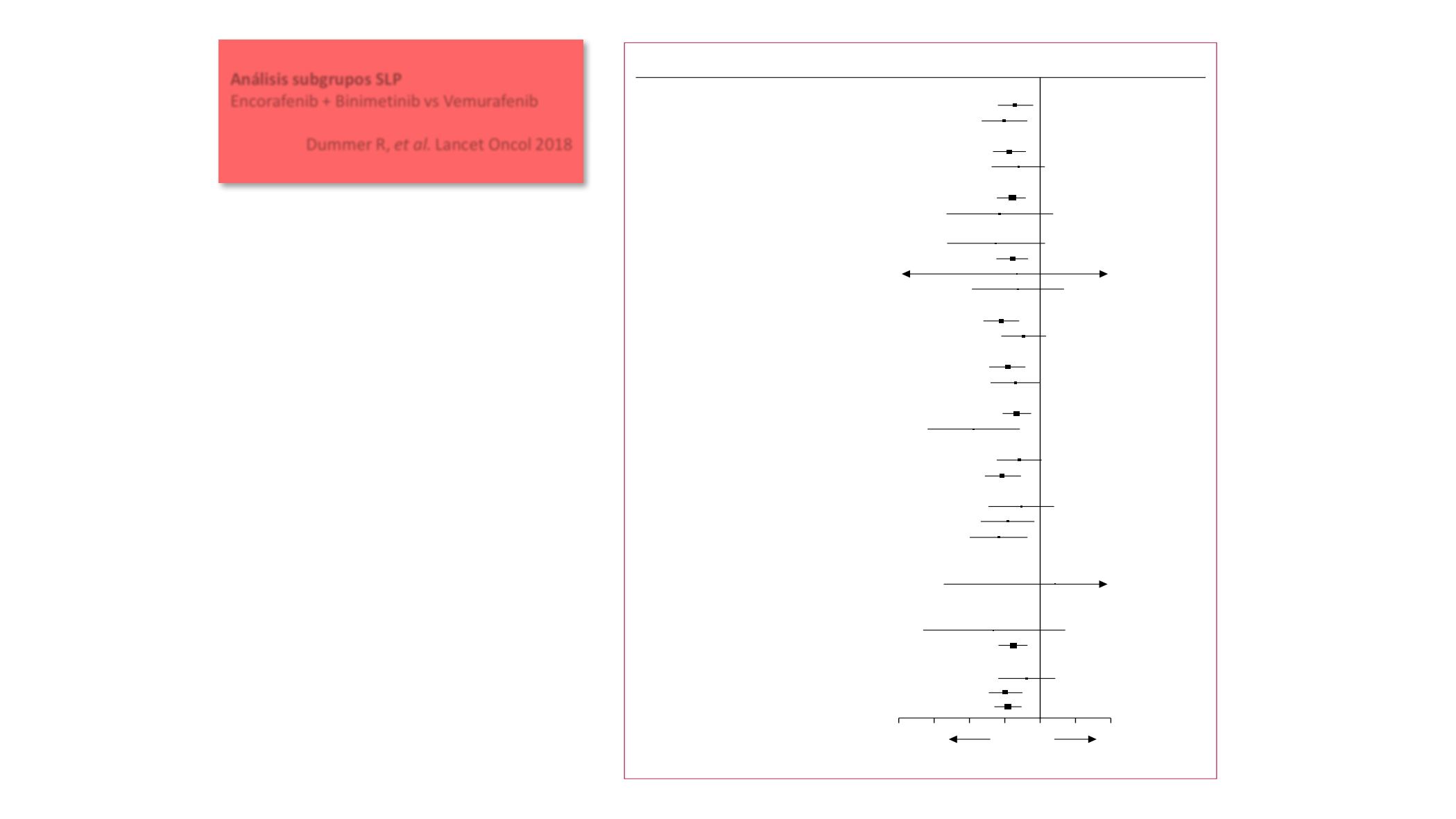
Figure 3:
Progression-free survival by prespecified subgroups according to baseline characteristics
Progression-free survival assessed by blinded independent central review. Comparisons are between the encorafenib
HR (95% CI)
Number of events/
number of patients
Sex
Male
Female
Age (years)
<65
≥65
Race
Caucasian
Non-Caucasian
Region
North America
Europe
Australia
Other
LDH concentration
<ULN
≥ULN
ECOG performance status
0
1
BRAF
mutation status
V600E
V600K
Disease stage
IIIB, IIIC, IVM1a, IVM1b
IVM1c
Number of organs involved at baseline
1
2
3
>3
Baseline brain metastases
Yes
No
Previous first-line immunotherapy
Yes
No
Previous adjuvant therapy
Yes
No
All patients
124/226
80/157
149/272
55/111
184/347
20/36
18/34
163/309
4/11
19/29
126/276
78/107
136/279
68/104
181/338
23/45
79/168
125/215
37/92
58/117
48/87
61/87
6/12
198/371
9/15
195/368
52/97
152/286
204/383
0·62 (0·44–0·89)
0·50 (0·32–0·79)
0·55 (0·40–0·77)
0·66 (0·39–1·12)
0·59 (0·44–0·79)
0·52 (0·19–1·43)
0·42 (0·16–1·10)
0·58 (0·42–0·79)
0·63 (0·06–6·97)
0·64 (0·26–1·60)
0·47 (0·33–0·67)
0·73 (0·47–1·14)
0·54 (0·38–0·76)
0·62 (0·38–1·01)
0·64 (0·48–0·85)
0·27 (0·11–0·68)
0·67 (0·43–1·04)
0·48 (0·34–0·69)
0·69 (0·36–1·32)
0·53 (0·31–0·89)
0·44 (0·25–0·78)
0·64 (0·39–1·06)
1·34 (0·15–11·78)
0·57 (0·43–0·75)
0·40 (0·10–1·64)
0·59 (0·44–0·78)
0·78 (0·45–1·35)
0·51 (0·37–0·71)
0·54 (0·41–0·71)
Favours encorafenib plus
binimetinib group
Favours
vemurafenib group
1·00
0·062
4·00
0·500
0·250
0·125
2·00
Análisis subgrupos SLP
Encorafenib + Binimetinib vs Vemurafenib
Dummer R,
et al.
Lancet Oncol 2018








