Extended 5-Year Follow-up Results of a Phase 1b Study
(BRIM7) of Cobimetinib Combined With Vemurafenib in
BRAF
V600
-Mutated Melanoma
Adil Daud,
1
Anna C. Pavlick,
2
Antoni Ribas,
3
Rene Gonzalez,
4
Karl D. Lewis,
4
Omid Hamid,
5
Thomas F. Gajewski,
6
Igor Puzanov,
7
Jessie J. Hsu,
8
Isabelle Rooney,
8
Erica Park,
8
Grant A. McArthur
9
1
University of California, San Francisco, San Francisco, CA, USA;
2
New York University Medical Center, New York, NY, USA;
3
Jonsson Comprehensive Cancer Center at University of California, Los Angeles, Los Angeles, CA, USA;
4
University of Colorado Comprehensive Cancer Center, Aurora, CO, USA;
5
The Angeles Clinic and Research Institute, Los Angeles, CA, USA;
6
The University of Chicago, Chicago, IL, USA;
7
Vanderbilt-Ingram Cancer Center, Nashville, TN, USA;
8
Genentech, Inc., South San Francisco, CA, USA;
9
Peter MacCallum Cancer Centre, East Melbourne, VIC, and University of Melbourne, Parkville, VIC, Australia
INTRODUCTION
•
BRAF
V600
is the driver of a significant proportion of melanomas, and targeted inhibition of
MAPK signaling is effective in treating advanced melanoma
1,2
– However, relapses frequently occur due to re-activation of MAPK signaling through
acquired mutations in pathway components
3,4
•
Combining the BRAF inhibitor (BRAFi) vemurafenib with the MEK inhibitor cobimetinib
has the potential to provide more effective tumor suppression
•
The phase Ib BRIM7 study (ClinicalTrials.gov ID, NCT01271803) evaluated the safety and
preliminary efficacy of cobimetinib combined with vemurafenib
5
•
A subsequent randomized phase 3 trial showed statistically significant and clinically meaningful
improvement in progression-free survival (PFS) and overall survival (OS) of cobimetinib
combined with vemurafenib compared with vemurafenib alone
6
•
Extended follow-up of this study provides useful insights on the long-term safety and
efficacy of cobimetinib combined with vemurafenib for patients with
BRAF
V600
-mutated
metastatic melanoma
OBJECTIVE
•
To evaluate the long-term efficacy and safety of cobimetinib combined with vemurafenib
after extended follow up in patients with
BRAF
V600
-mutated metastatic melanoma (data cutoff
July 10, 2017)
METHODS
Study Design
•
BRIM7 was an open-label, multicenter, phase 1b dose-escalation study conducted in
two stages (dose escalation and expansion)
•
In the dose-escalation stage, patients received cobimetinib at 60, 80, or 100 mg once daily
(QD) for 14 days on/14 days off (14/14); 21 days on/7 days off (21/7); or continuously (28/0),
combined with vemurafenib at 720 or 960 mg twice daily (BID) continuously (
Figure 1
)
•
Two dose levels were expanded: cobimetinib (60 mg QD 21/7) and vemurafenib (720 and
960 mg BID)
•
Treatment was continued until disease progression, unacceptable toxicity, or withdrawal
of consent
Figure 1.
Study design.
Cobimetinib 60 mg QD
21/7 days on/off
+
Vemurafenib 720 mg BID
28/0 days on/off
Cohort Expansion Stage
(2 cohorts)
Dose Escalation Stage
3 + 3 design (10 cohorts)
Follow-up Period
(2 cohorts)
Cobimetinib 60, 80, or
100 mg QD
14/14, 21/7, or
28/0 days on/off
+
Vemurafenib 720 or
960 mg BID
28/0 days on/off
All patients
Cobimetinib 60 mg QD
21/7 days on/off
+
Vemurafenib 960 mg BID
28/0 days on/off
Prior
treatment
with
vemurafenib
n = 66
No prior
BRAFi
treatment
n = 63
BID, twice daily; BRAFi, BRAF inhibitor; QD, once daily.
Key Eligibility Criteria
Efficacy
•
Confirmed BORR in BRAFi-naive patients and in Vem-PD patients was unchanged from
previous reports
7,8
(
Table 2
)
Table 2.
Confirmed BORR in Each Cohort
Vem-PD
n = 66
BRAFi-Naive
n = 63
Objective response, % (95% CI)
15.2 (7.5–25.5)
87.3 (76.7–94.4)
Complete response
1.5
19.0
Partial response
13.6
68.3
Stable disease
42.4
9.5
Progressive disease
36.4
3.2
Not assessable/not done
6.0
0
BORR, best overall response rate; BRAFi, BRAF inhibitor; CI, confidence interval; Vem-PD, vemurafenib progressor.
•
Median PFS for Vem-PD and BRAFi-naive patients remained unchanged from previous
reports, at 2.8 months and 13.8 months, respectively (
Figure 2
)
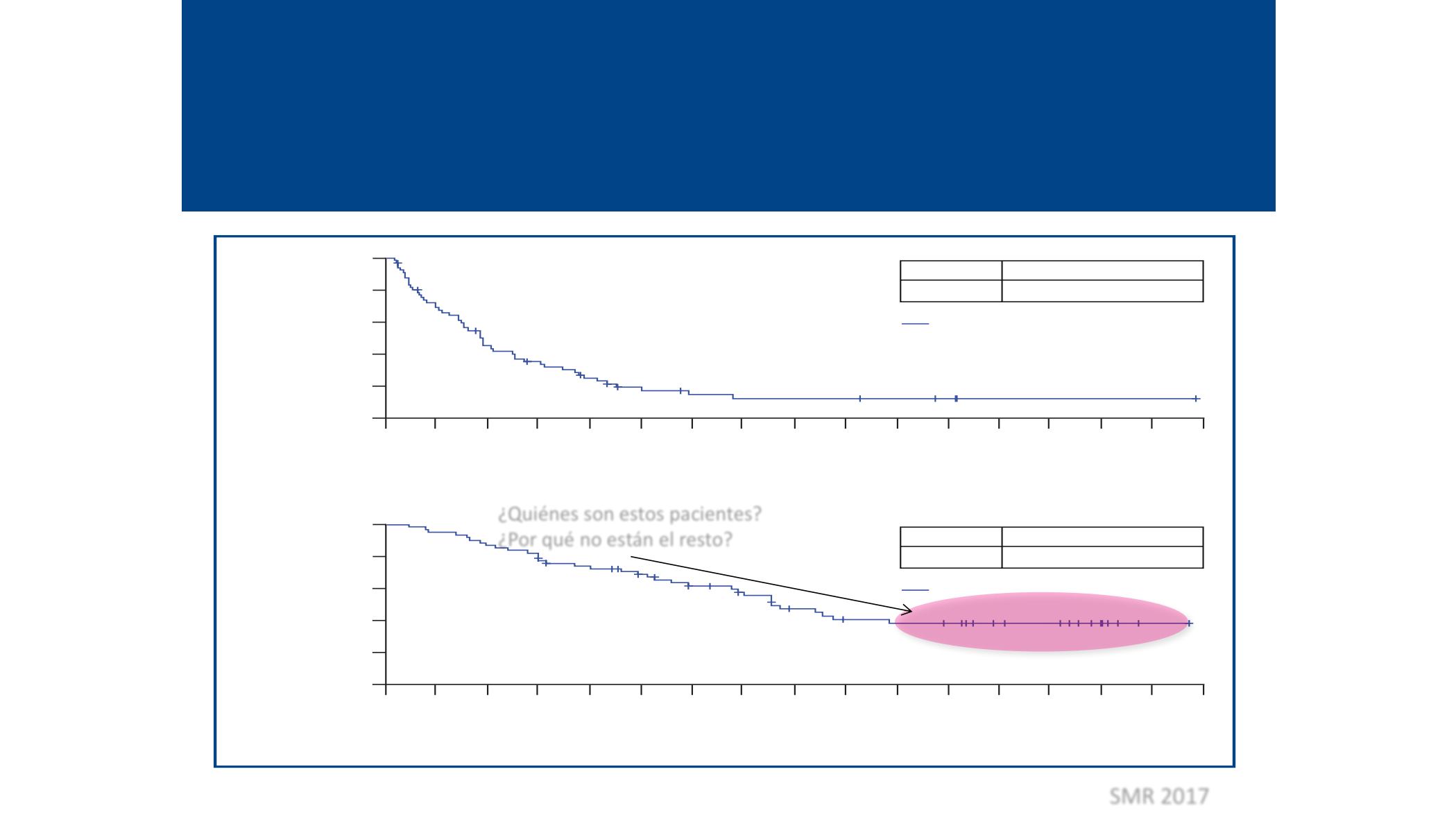
Figure 2.
Kaplan-Meier curves for PFS in (A) Vem-PD patients and
(B) BRAFi-naive patients.
100
80
60
40
0
20
BRAFi-naive
63 55 42 32 26 20 16 14 13 12 9
4
2
8 5
1
9 5
13
21
29
37
45
53
61
17
25
33
41
49
57
65
No. of Patients at Risk
Time, Months
B
Events, n
45
MedianPFS,months (95%CI)
13.8 (10.8–20.6)
+
BRAFi-naive (n = 63)
Censored
100
80
60
40
0
20
Vemurafenib
progressors
64 15 7
2
2 2
1 1
1 1 —
— —
— —
1
9 5
13
21
29
37
45
53
61
17
25
33
41
49
57
65
No. of Patients at Risk
Time, Months
A
Events, n
61
MedianPFS,months (95%CI)
2.8 (2.6–3.4)
+
Vemurafenib progressors (n = 66)
Censored
PFS,%
PFS,%
BRAFi, BRAF inhibitor; CI, confidence interval; PFS, progression-free survival; Vem-PD, vemurafenib progressor.
•
Median OS for BRAFi-naive patients increased from 31.2 months at 4 years’ follow-up
(data cutoff April 25, 2016)
8
to 31.8 months at the latest data cutoff (July 10, 2017),
while landmark OS rates reached a plateau at 39.2% (
Figure 3
and
Table 3
)
•
Median OS for Vem-PD patients remained unchanged from previous reports at 8.5 months,
and landmark OS rates were stable
Figure 3.
Kaplan-Meier curves for OS in (A) Vem-PD patients and (B) BRAFi-naive
patients.
100
80
60
40
20
OS,%
A
Events, n
53
MedianOS,months (95%CI)
8.5 (6.7–11.1)
+
Vemurafenib progressors (n = 66)
Censored
Table 5.
Adverse Events (AEs), Regardless of Attribution, in ≥20% of
Safety-Evaluable Patients, and AESI, in BRIM7 at 5 Years
Common Treatment-EmergentAEs, n (%)
Vem-PD
n = 66
BRAFi-Naive
n = 63
Any
Grade
Grade
≥3
Any
Grade
Grade
≥3
Non-acneiform rash
a
25 (38)
1 (2)
56 (89)
9 (14)
Diarrhea
31 (47)
2 (3)
52 (83)
6 (10)
Fatigue
18 (27)
1 (2)
46 (73)
7 (11)
Photosensitivity
a
22 (33)
1 (2)
46 (73)
1 (2)
Elevations in LFTs
a
22 (33)
4 (6)
44 (70)
13 (21)
Nausea
23 (35)
2 (3)
37 (59)
2 (3)
Arthralgia
8 (12)
1 (2)
31 (49)
7 (11)
Vomiting
14 (21)
1 (2)
30 (48)
0
CPK level elevation
10 (15)
1 (2)
30 (48)
2 (3)
Pyrexia
11 (17)
1 (2)
28 (44)
1 (2)
Acneiform rash
a
9 (14)
1 (2)
28 (44)
2 (3)
Peripheral edema
11 (17)
0
27 (43)
0
Anemia
11 (17)
5 (8)
23 (37)
7 (11)
Blood creatinine increased
6 (9)
0
21 (33)
1 (2)
Myalgia
4 (6)
0
20 (32)
1 (2)
Headache
13 (20)
0
19 (30)
2 (3)
Pruritus
7 (11)
0
19 (30)
1 (2)
Hypertension
6 (9)
1 (2)
19 (30)
5 (8)
Actinic keratosis
3 (5)
0
19 (30)
0
Decreased appetite
14 (21)
0
17 (27)
0
Chills
10 (15)
0
17 (27)
0
Dry skin
2 (3)
0
17 (27)
0
Upper respiratory tract infection
6 (9)
0
16 (25)
0
Hypokalemia
4 (6)
0
16 (25)
3 (5)
Seborrheic keratosis
1 (2)
0
15 (24)
1 (2)
Skin papilloma
0
0
15 (24)
0
Blurred vision
2 (3)
0
14 (22)
0
Abdominal pain
10 (15)
1 (2)
13 (21)
0
Constipation
10 (15)
1 (2)
13 (21)
0
Cough
6 (9)
0
13 (21)
0
Hypophosphatemia
3 (5)
3 (5)
13 (21)
6 (10)
Selected AESI, n (%)
Retinal detachment/retinopathy (grade ≥2)
a
0
0
3 (5)
0
Retinal vein occlusion (any grade)
0
0
0
0
QTc prolongation (grade ≥3)
a
— 2 (3)
— 4 (6)
cuSCC (any grade)
a
5 (8)
5 (8)
8 (13)
8 (13)
AESI, adverse events of special interest; CPK, creatine phosphokinase; cuSCC, cutaneous squamous cell carcinoma;
LFTs, liver function tests; Vem-PD, vemurafenib progressor.
a
Grouped terms.
t
0
Mutation
ersion 1.1
, tolerability
ition of the
ls
n
ks after
BRAFi, BRAF inhibitor; CI, confidence interval; PFS, progression-free survival; Vem-PD, vemurafenib progressor.
•
Median OS for BRAFi-naive patients increased from 31.2 months at 4 years’ follow-up
(data cutoff April 25, 2016)
8
to 31.8 onths at the latest data cutoff (July 10, 2017),
while landmark OS rates reached a plateau at 39.2% (
Figure 3
and
Table 3
)
•
Median OS for Vem-PD patients remained unchanged from previous reports at 8.5 months,
and landmark OS rates were stable
Figure 3.
Kaplan-Meier curves for OS in (A) Vem-PD patients and (B) BRAFi-naive
patients.
100
80
60
40
0
20
BRAFi-naive
63 60 55 48 44 39 33 28 22 18 17
10
1 —
5
16 11
1
9 5
13
21
29
37
45
53
61
17
25
33
41
49
57
65
OS, %
No. of Patients at Risk
Time, Months
B
Events, n
34
Median OS, months (95% CI)
31.8 (24.5–NE)
+
BRAFi-naive (n = 63)
Censored
100
80
60
40
0
20
Vemurafenib
progressors
66 44 28 21 14 8
6 5
5 5
4
1
1 —
1
3 1
1
9 5
13
21
29
37
45
53
61
17
25
33
41
49
57
65
OS, %
No. of Patients at Risk
Time, Months
A
Events, n
53
Median OS, months (95% CI)
8.5 (6.7–11.1)
+
Vemurafenib progressors (n = 66)
Censored
BRAFi, BRAF inhibitor; CI, confidence interval; NE, not estimable; OS, overall survival; Vem-PD, vemurafenib progressor.
Constipati
Cough
Hypophos
Selected A
Retinal det
Retinal vei
QTc prolon
cuSCC (an
AESI, adverse e
LFTs, liver functi
a
Grouped terms
Table 6.
Tr
Treatment
Vem-PD p
Dose int
reductio
Permane
BRAFi-nai
Dose int
reductio
Permane
AE, adverse ev
¿Quiénes son estos pacientes?
¿Por qué no están el resto?
SMR 2017








