Cobimetinib combined with vemurafenib in advanced
BRAF
V600
-mutant melanoma (coBRIM): updated efficacy
results from a randomised, double-blind, phase 3 trial
Paolo A Ascierto, Grant A McArthur, Brigitte Dréno, Victoria Atkinson, Gabrielle Liszkay, Anna Maria Di Giacomo, Mario Mandalà, Lev Demidov,
Daniil Stroyakovskiy, Luc Thomas, Luis de la Cruz-Merino, Caroline Dutriaux, Claus Garbe, Yibing Yan, MatthewWongchenko, Ilsung Chang,
Jessie J Hsu, Daniel O Koralek, Isabelle Rooney, Antoni Ribas, James Larkin
Summary
Background
The combination of cobimetinib with vemurafenib improves progression-free survival compared with
placebo and vemurafenib in previously untreated patients with
BRAF
V600
-mutant advanced melanoma, as previously
reported in the coBRIM study. In this Article, we report updated efficacy results, including overall survival and safety
after longer follow-up, and selected biomarker correlative studies.
Methods
In this double-blind, randomised, placebo-controlled, multicentre study, adult patients (aged ≥18 years)
with histologically confirmed
BRAF
V600
mutation-positive unresectable stage IIIC or stage IV melanoma were
randomly assigned (1:1) using an interactive response system to receive cobimetinib (60 mg once daily for 21 days
followed by a 7-day rest period in each 28-day cycle) or placebo, in combination with oral vemurafenib (960 mg
twice daily). Progression-free and overall survival were primary and secondary endpoints, respectively; all analyses
were done on the intention-to-treat population. This study is registered with ClinicalTrials.gov, number
NCT01689519, and is ongoing but no longer recruiting participants.
Findings
Between Jan 8, 2013, and Jan 31, 2014, 495 eligible adult patients were enrolled and randomly assigned to
the cobimetinib plus vemurafenib group (n=247) or placebo plus vemurafenib group (n=248). At a median
follow-up of 14·2 months (IQR 8·5–17·3), the updated investigator-assessed median progression-free survival was
12·3 months (95% CI 9·5–13·4) for cobimetinib and vemurafenib versus 7·2 months (5·6–7·5) for placebo and
vemurafenib (HR 0·58 [95% CI 0·46–0·72], p<0·0001). The final analysis for overall survival occurred when
255 (52%) patients had died (Aug 28, 2015). Median overall survival was 22·3 months (95% CI 20·3–not estimable)
for cobimetinib and vemurafenib versus 17·4 months (95% CI 15·0–19·8) for placebo and vemurafenib (HR 0·70,
95% CI 0·55–0·90; p=0·005). The safety profile for cobimetinib and vemurafenib was tolerable and manageable,
and no new safety signals were observed with longer follow-up. The most common grade 3–4 adverse events
occurring at a higher frequency in patients in the cobimetinib and vemurafenib group compared with the
vemurafenib group were γ-glutamyl transferase increase (36 [15%] in the cobimetinib and vemurafenib group
vs
25 [10%] in the placebo and vemurafenib group), blood creatine phosphokinase increase (30 [12%]
vs
one [<1%]),
and alanine transaminase increase (28 [11%]
vs
15 [6%]). Serious adverse events occurred in 92 patients (37%) in
the cobimetinib and vemurafenib group and 69 patients (28%) in the vemurafenib group. Pyrexia (six patients
[2%]) and dehydration (five patients [2%]) were the most common serious adverse events reported in the
cobimetinib and vemurafenib group. A total of 259 patients have died: 117 (47%) in the cobimetinib and
vemurafenib group and 142 (58%) in the vemurafenib group. The primary cause of death was disease progression
in most patients: 109 (93%) of 117 in the cobimetinib and vemurafenib group and 133 (94%) of 142 in the
vemurafenib group.
Interpretation
These data confirm the clinical benefit of cobimetinib combined with vemurafenib and support the use
of the combination as a standard first-line approach to improve survival in patients with advanced
BRAF
V600
-mutant
Lancet Oncol
2016; 17: 1248–60
Published
Online
July 29, 2016
http://dx.doi.org/10.1016/S1470-2045(16)30122-X
See
Comment
page 1178
Istituto NazionaleTumori
Fondazione G Pascale, Naples,
Italy
(P A Ascierto MD)
; Peter
MacCallumCancer Centre,
East Melbourne,VIC, Australia
(Prof G A McArthur FRACP)
;
University of Melbourne,
Parkville,VIC, Australia
(Prof G A McArthur)
; Nantes
University, Nantes, France
(Prof B Dréno MD)
;
Princess Alexandra Hospital,
Woolloongabba, QLD, Australia
(V Atkinson MD)
; National
Institute of Oncology,
Budapest, Hungary
(G Liszkay MD)
; Azienda
Ospedaliera Universitaria
Senese, Siena, Italy
(A M Di Giacomo MD)
;
Papa Giovanni XXIII Hospital,
Bergamo, Italy
(MMandalà MD)
; N N Blokhin
Russian Cancer Research
Center, Moscow, Russia
(L Demidov MD)
; Moscow City
Oncology Hospital 62,
Krasnogorsk, Russia
(D Stroyakovskiy MD)
; Centre
Hospitalier Lyon Sud,
Lyon 1 University, Lyon, France
(Prof LThomas MD)
; Lyons
Cancer Research Center, Lyon,
France
(Prof LThomas)
;
Hospital UniversitarioVirgen
Macarena, Seville, Spain
(L de la Cruz-Merino MD)
;
1248
www.thelancet.com/oncologyVol 17 September 2016
Downloaded from ClinicalKey.com at Fundacion Marques de Valdecilla October 23, 2016.
For personal use only. No other uses without permission. Copyright ©2016. Elsevier Inc. All rights reserved.
Articles
and by comparing
below the media
proportional hazar
This study is regi
NCT01689519. The
and patients alrea
followed for long-te
Role of the funding
F Hoffmann-La
istered, and sponso
the academic aut
representatives. D
analysed in collabo
vouch for the accur
IC, JJH, MW, DOK
data. JL, GAM, PA
the first draft of the
subsequent drafts
A
Number at risk
Cobimetinib and
vemurafenib group
Placebo and
vemurafenib group
0
3
6
9
12
15
18
21
24
247
248
Placebo and
vemurafenib
(n=248)
Cobimetinib and
vemurafenib
(n=247)
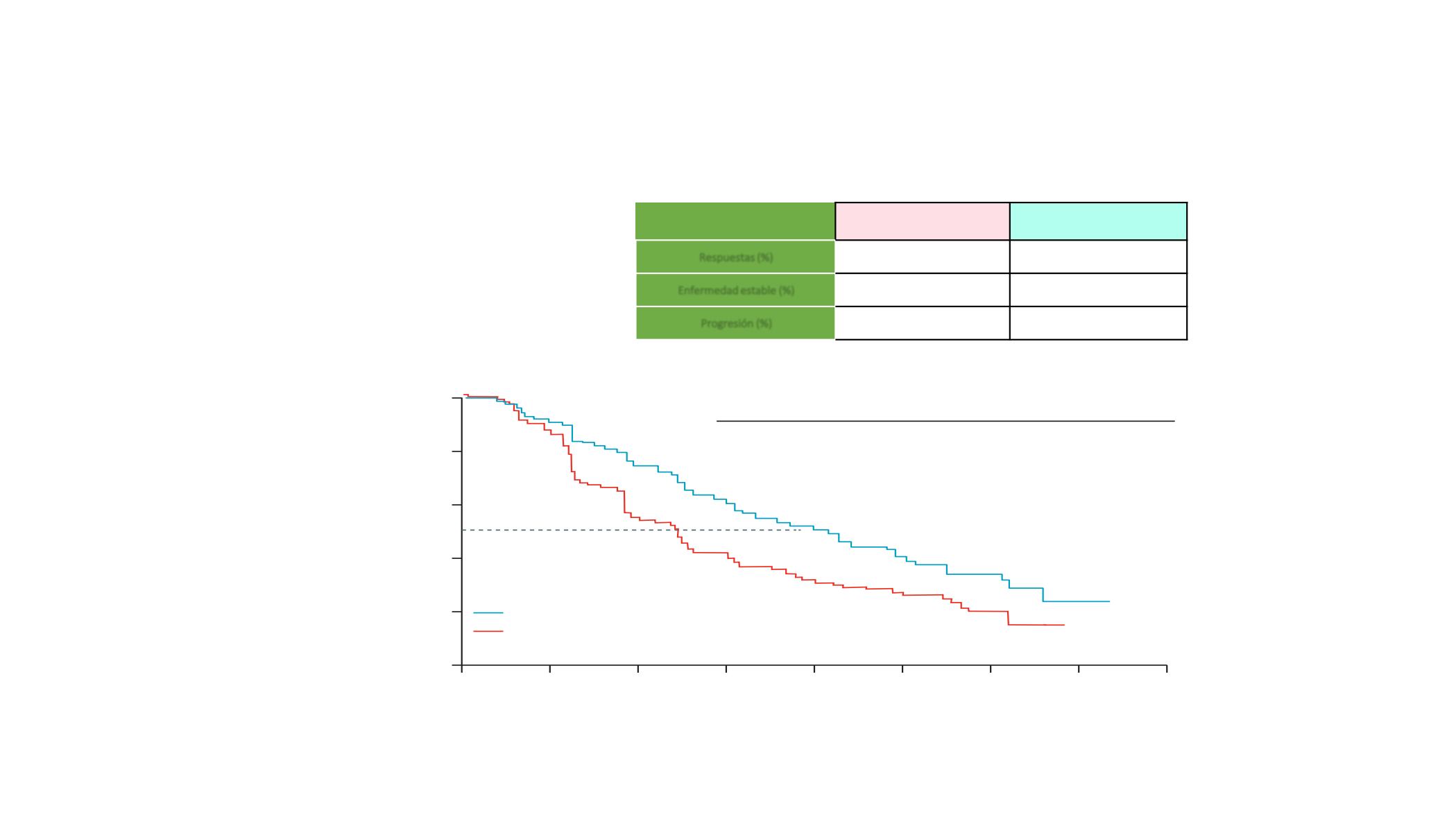
Events, n (%)
M dian progression-free
survival, mo ths (95% CI)
Hazard ratio (95% CI)
180 (72·6%)
7·2 (5·6–7·5)
0·58 (0·46–0·72); p<0·0001
143 (57·9%)
12·3 (9·5–13·4)
215
205
174
120
46
30
19
13
1
0
··
··
142
87
104
54
0
20
40
60
80
100
Progression-free survival (%)
+
+
Cobimetinib and vemurafenib group
Placebo and vemurafenib group
Censored patients
+
++++ ++++++++++++++++++++++
+
+
+ + + +
+
++
++++
++
+
++
+ +
+ + + +++ + + +
++ ++++++++++
++
++++
+
++
+
Eficacia
Placebo + Vemurafenib
Cobimetinib + Vemurafenib
Respuest s (%)
50
70
Enfermedad estable (%)
37
18
Progresión (%)
10
8








