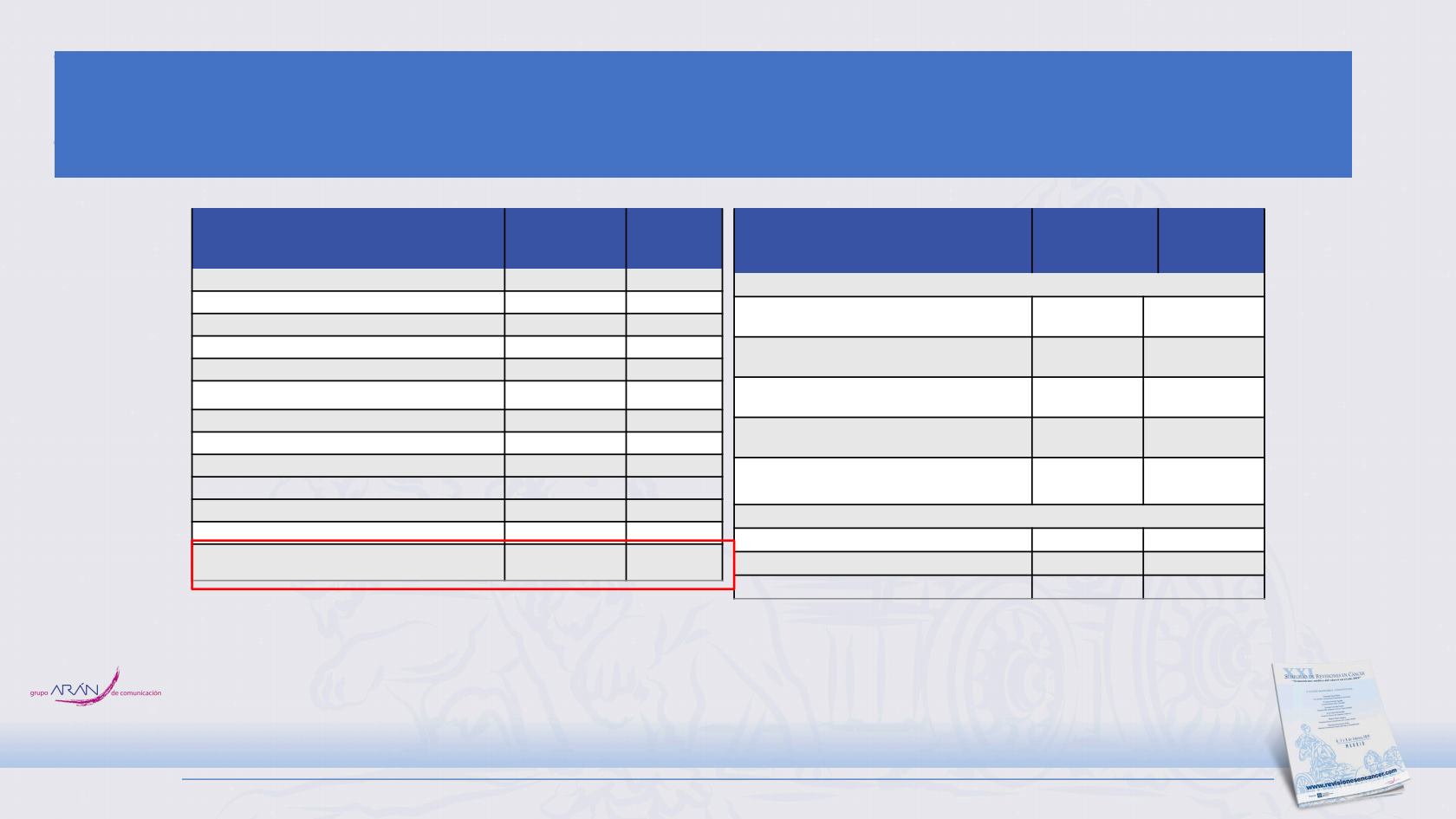
Baseline Patient Characteristics
Characteristic
Atezolizumab +
CP/ET
(N = 201)
Placebo
+ CP/ET
(N = 202)
Median age (range) years
64 (28
-
90)
64 (26
-
87)
Age group — no. (%)
< 65 years
111 (55.2)
106 (52.5)
≥ 65 years
90 (44.8)
96 (47.5)
Male sex, n (%)
a
129 (64.2)
132 (65.3)
ECOG performance status — no. (%)
a
0
73 (36.3)
67 (33.2)
1
128 (63.7)
135 (66.8)
Smoking status — no. (%)
Never smoked
9 (4.5)
3 (1.5)
Current smoker
74 (36.8)
75 (37.1)
Former smoker
118 (58.7)
124 (61.4)
Brain metastases at enrolment
— no. (%)
a
17 (8.5)
18 (8.9)
Characteristic
Atezolizumab +
CP/ET
(N = 201)
Placebo
+ CP/ET
(N = 202)
Blood-based tumour mutational burden — no. /total (%)
b
< 10 mutations/Mb
71/173 (41.0) 68/178
(38.2)
≥ 10 mutations/Mb
102/173 (59.0) 110/178
(61.8)
< 16 mutations/Mb
133/173 (76.9) 138/178
(77.5)
≥ 16 mutations/Mb
40/173 (23.1) 40/178
(22.5)
Median sum of longest diameter of target
lesions at baseline (range)
113.0
(12.0–
325.0)
105.5
(15.0–
353.0)
Previous anti-cancer treatments — no. (%)
Chemotherapy or non-anthracycline
8 (4.0)
12 (5.9)
Radiotherapy
25 (12.4)
28 (13.9)
Cancer-related surgery
33 (16.4)
25 (12.4)
Clinical data cut-off date: April 24, 2018, 11 months after the last patient was enrolled. CI, confidence interval. HR, hazard ratio; CP/ET, carboplatin + etoposide.
Adapted from The New England Journal of Medicine, Horn L, et al, First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer, Epub ahead of








