2
Safety Summary
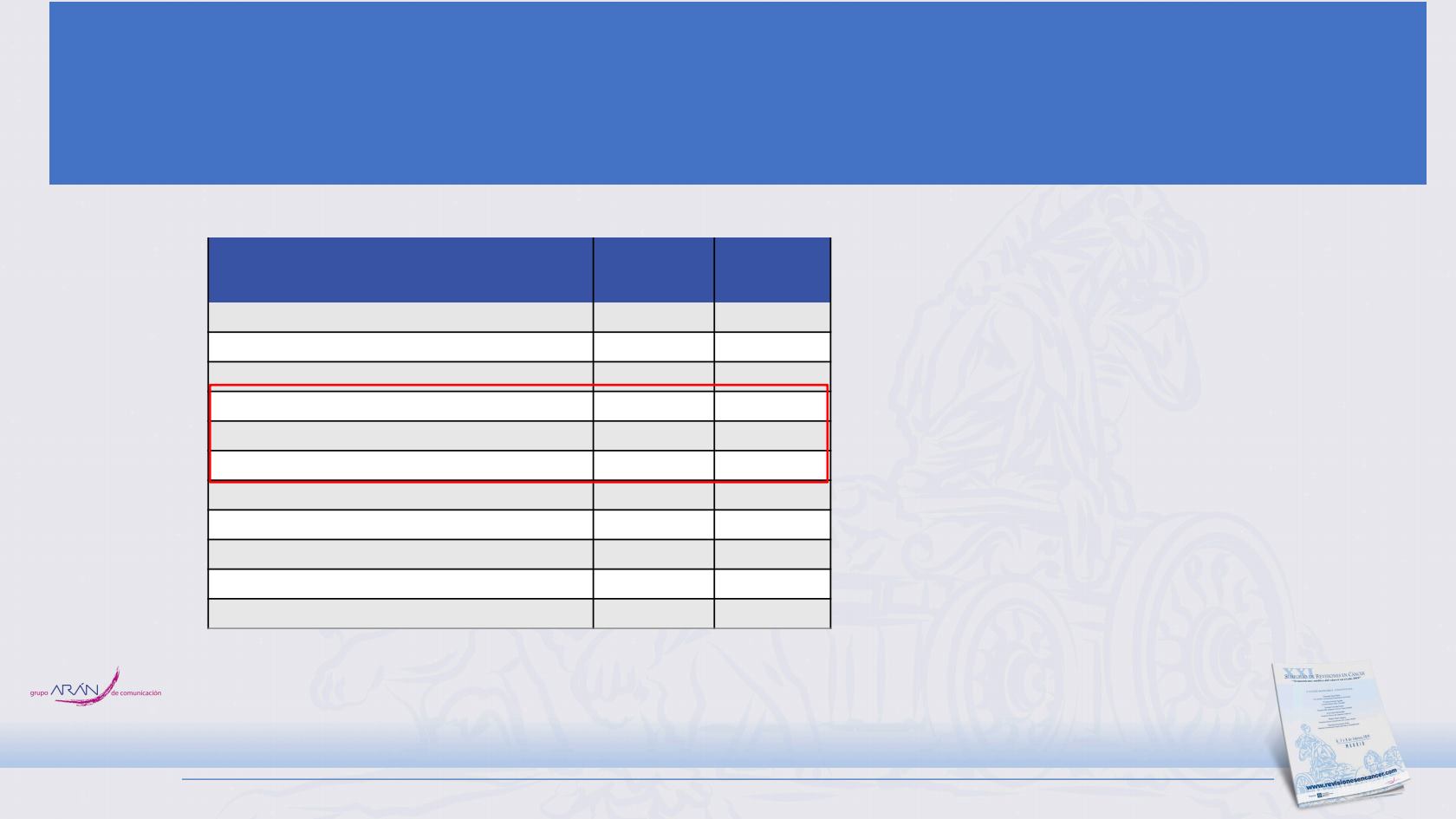
Patients — no. (%)
Atezolizumab +
CP/ET
(N = 198)
a
Placebo +
CP/ET
(N = 196)
a
Patients with ≥ 1 AE
198 (100)
189 (96.4)
Grade 3–4 AEs
133 (67.2)
125 (63.8)
Grade 5 AEs
4 (2.0)
11 (5.6)
Treatment-related AEs
b
188 (94.9)
181 (92.3)
Treatment-related Grade 3–4 AEs
112 (56.6)
110 (56.1)
Treatment-related Grade 5 AEs
3 (1.5)
3 (1.5)
Serious AEs
74 (37.4)
68 (34.7)
Treatment-related serious AEs
b
45 (22.7)
37 (18.9)
AEs leading to withdrawal from any treatment
b
22 (11.1)
6 (3.1)
AEs leading to withdrawal from carboplatin
5 (2.5)
1 (0.5)
AEs leading to withdrawal from etoposide
8 (4.0)
2 (1.0)
•
Median duration of treatment (range):
–
Atezolizumab: 4.7 months (0–21)
–
Median duration of chemotherapy (range) in
atezolizumab + CP/ET vs. placebo + CP/ET
–
Carboplatin: 2.3 (0–4) vs. 2.2 months (0–4)
–
Etoposide: 2.3 (0–4) vs. 2.2 months (0–4)
•
Median no. of treatment doses (range):
–
Atezolizumab: 7 (1–30)
•
Median no. of doses of chemotherapy (range) in
atezolizumab + CP/ET vs. placebo + CP/ET:
–
Carboplatin: 4 (1–6) vs. 4 (1–5)
–
Etoposide: 12 (1–18) vs. 12 (2–15)
Clinical data cut-off date: April 24, 2018, 11 months after the last patient was enrolled. CI, confidence interval. HR, hazard ratio; CP/ET, carboplatin + etoposide.
Adapted from The New England Journal of Medicine, Horn L, et al, First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer, Epub ahead of
print.








