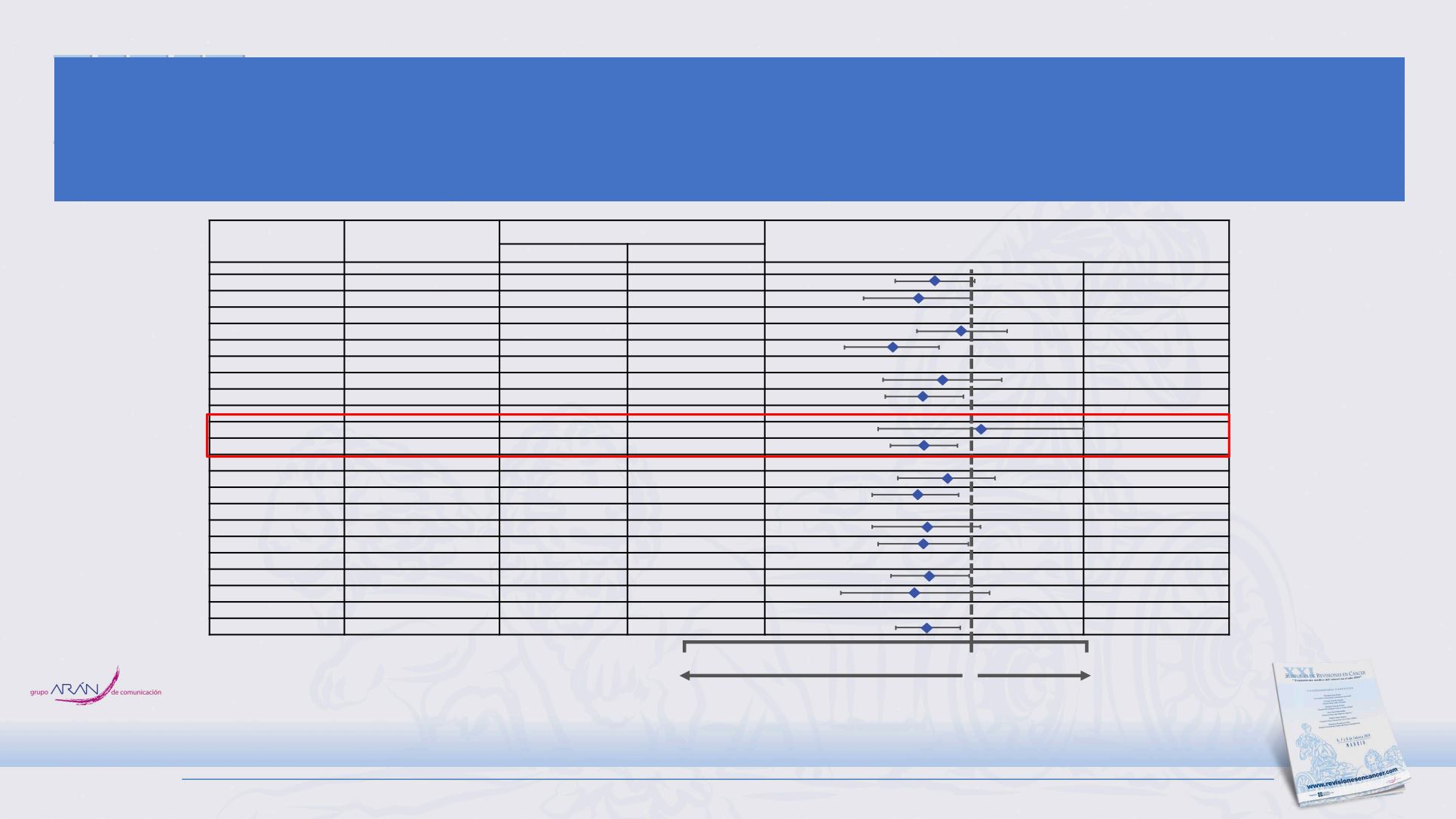
OS by Subgroup
Subgroup
No. of patients (%)
Median overall survival (months)
Hazard ratio for death (95% CI)
a
Placebo
Atezolizumab
Male
261 (65)
10.9
12.3
0.74 (0.54, 1.02)
Female
142 (35)
9.5
12.5
0.65 (0.42, 1.00)
< 65 years
217 (54)
11.5
12.1
0.92 (0.64, 1.32)
≥ 65 years
186 (46)
9.6
12.5
0.53 (0.36, 0.77)
ECOG PS 0
140 (35)
12.4
16.6
0.79 (0.49, 1.27)
ECOG PS 1
263 (65)
9.3
11.4
0.68 (0.50, 0.93)
Brain metastases
35 (9)
9.7
8.5
1.07 (0.47, 2.43)
No brain metastases
368 (91)
10.4
12.6
0.68 (0.52, 0.89)
Liver metastases
149 (37)
7.8
9.3
0.81 (0.55, 1.20)
No liver metastases
254 (63)
11.2
16.8
0.64 (0.45, 0.90)
bTMB < 10 mut/mb
139 (34)
9.2
11.8
0.70 (0.45, 1.07)
bTMB ≥ 10 mut/mb
212 (53)
11.2
14.6
0.68 (0.47, 0.97)
bTMB < 16 mut/mb
271 (67)
9.9
12.5
0.71 (0.52, 0.98)
bTMB ≥ 16 mut/mb
80 (20)
11.9
17.8
0.63 (0.35, 1.15)
ITT (N = 403)
403 (100)
10.3
12.3
0.70 (0.54, 0.91)
0.1
1.0
2.5
Atezolizumab better Placebo better
Clinical data cut-off date: April 24, 2018, 11 months after the last patient was enrolled. CI, confidence interval. HR, hazard ratio; CP/ET, carboplatin + etoposide.
Adapted from The New England Journal of Medicine, Horn L, et al, First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer, Epub ahead of
print.








