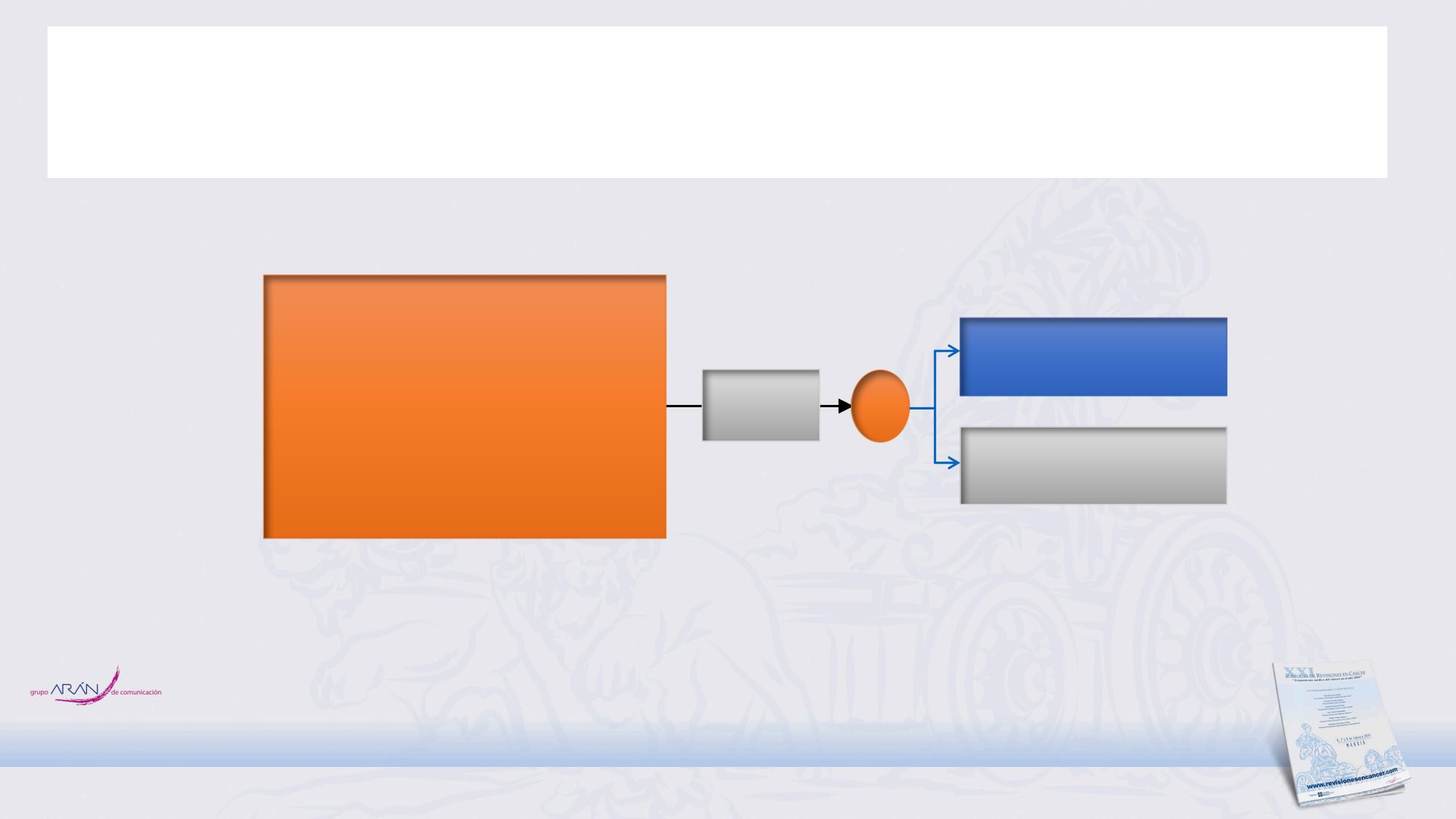
NCT02046733 / STIMULI: Phase II Ipilimumab with nivolumab vs Observation
for 1L LD-SCLC after chemo-radiotherapy
* ≤ 1 for enrollment, ≤ 2 for randomization.
†
Except fatigue, appetite, esophagitis, and renal impairment (≤ Grade 2 allowed) and alopecia (any grade allowed).
AE, adverse event; CRT, chemoradiotherapy; ECOG PS, Eastern Cooperative Oncology Group Performance Status;
LD, limited disease; OR, objective response; OS, overall survival
;
N=260
•
Primary outcome measure: OS, PFS
•
Secondary outcome measures: OR, TTF, AEs
Start Date: July 2014
Estimated Study Completion Date:
January 2022
Estimated Primary Completion Date:
October 2019
Squibb and others
Ipilimumab + nivolumab
R
Observation
Key Inclusion Criteria
≥18 years of age
Untreated LD-SCLC
ECOG PS ≤1 or 2*
Non PD after completion of CRT, PCI
Adequate hematological, renal, and liver
function
Recovery of all AEs to Grade ≤1
†
CRT + PCI
Objetive: # randomized patients: 260
325 required
Current recruitment 124








