2
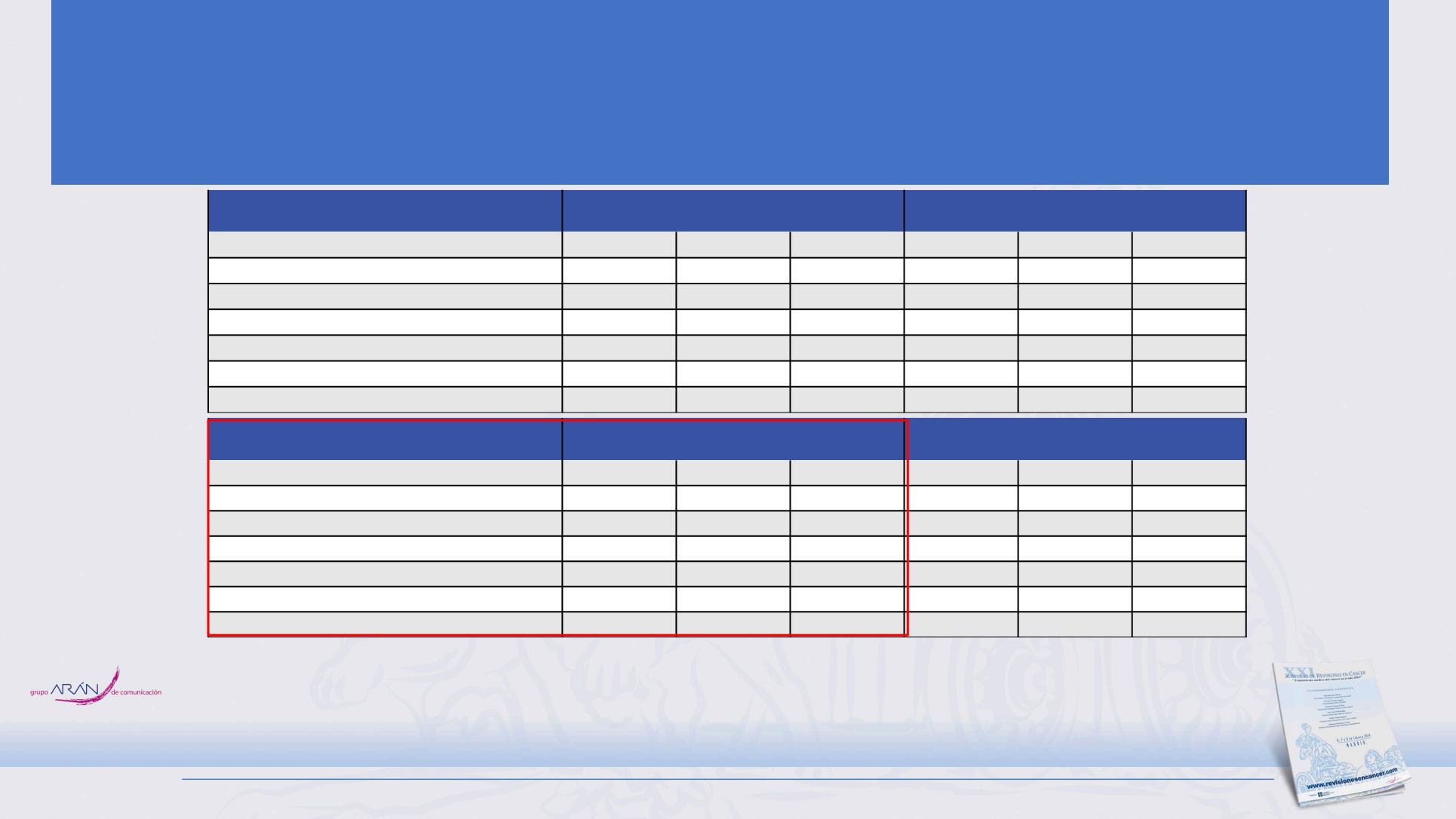
Most Frequent AEs
Clinical data cut-off date: April 24, 2018, 11 months after the last patient was enrolled. CI, confidence interval. HR, hazard ratio; CP/ET, carboplatin + etoposide.
Adapted from The New England Journal of Medicine, Horn L, et al, First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer, Epub ahead of
print.
Treatment-related AEs — no. (%)
> 5% Grade 3–4 AEs in either treatment group
Atezolizumab + CP/ET
(N = 198)
a
Placebo + CP/ET
(N = 196)
a
Grade 1–2 Grade 3–4 Grade 5 Grade 1–2 Grade 3–4 Grade 5
Neutropenia
26 (13.1)
45 (22.7)
1 (0.5)
20 (10.2)
48 (24.5)
0
Anaemia
49 (24.7)
28 (14.1)
0
41 (20.9)
24 (12.2)
0
Neutrophil count decreased
7 (3.5)
28 (14.1)
0
12 (6.1)
33 (16.8)
0
Thrombocytopenia
12 (6.1)
20 (10.1)
0
14 (7.1)
15 (7.7)
0
Leukopenia
15 (7.6)
10 (5.1)
0
10 (5.1)
8 (4.1)
0
Febrile neutropenia
0
6 (3.0)
0
0
12 (6.1)
0
Immune-related AEs — no. (%)
> 1% Grade 3–4 AEs in either treatment group
Atezolizumab + CP/ET
(N = 198)
a
Placebo + CP/ET
(N = 196)
a
Grade 1–2 Grade 3–4 Grade 5 Grade 1–2 Grade 3–4 Grade 5
Rash
33 (16.7)
4 (2.0)
0
20 (10.2)
0
0
Hepatitis
11 (5.6)
3 (1.5)
0
9 (4.6)
0
0
Infusion-related reaction
7 (3.5)
4 (2.0)
0
9 (4.6)
1 (0.5)
0
Pneumonitis
3 (1.5)
1 (0.5)
0
3 (1.5)
2 (1.0)
0
Colitis
1 (0.5)
2 (1.0)
0
0
0
0
Pancreatitis
0
1 (0.5)
0
0
2 (1.0)
0








