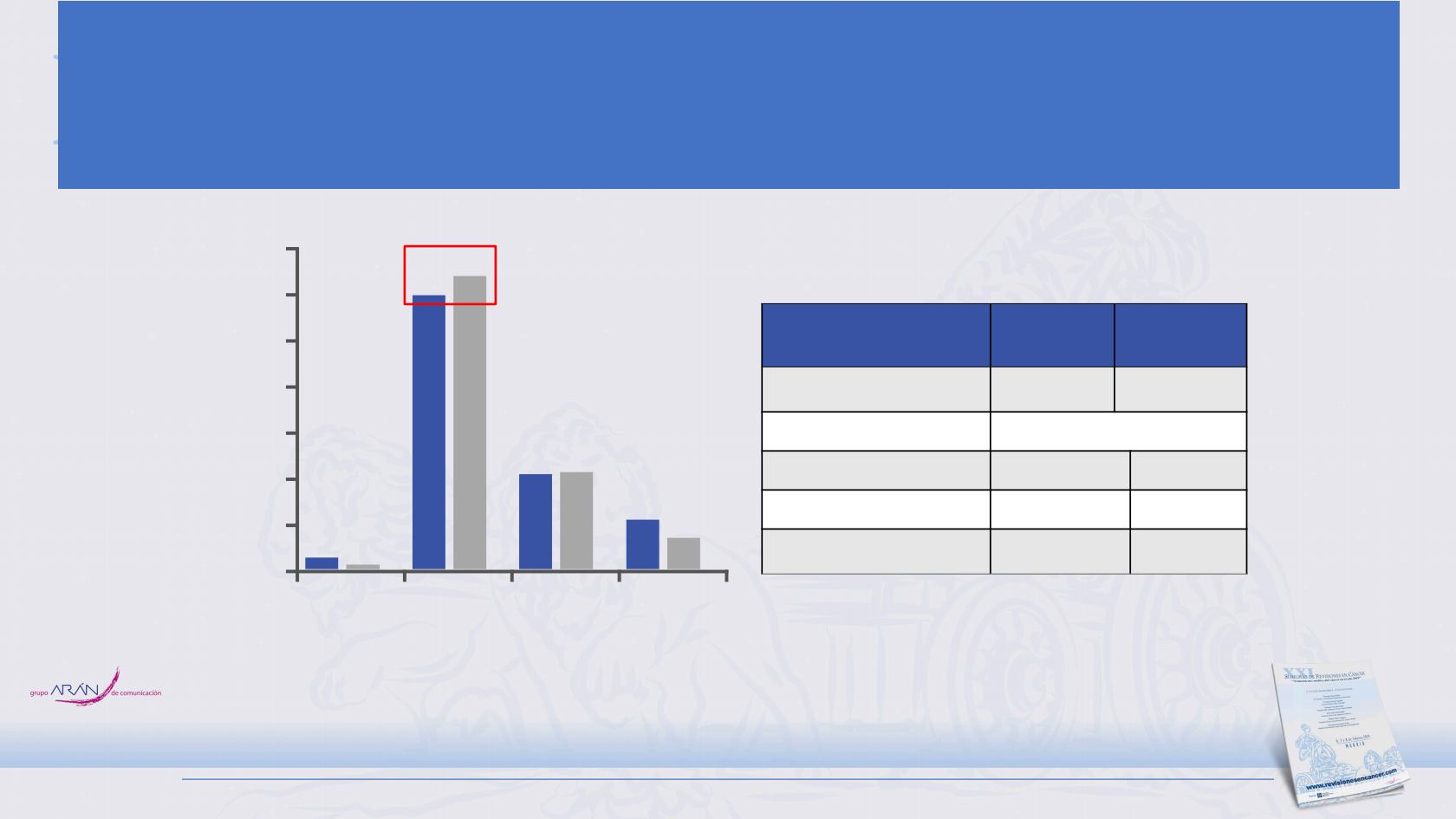
ORR and DoR
2,5
60,2
20,9
10,9
1,0
64,4
21,3
6,9
CR
CR/PR
SD
PD
70
60
50
40
30
20
10
0
Response (%)
Duration of response
Atezolizumab
+ CP/ET
(N = 121)
Placebo
+ CP/ET
(N = 130)
Median duration — months
(range)
4.2
(1.4
a
to 19.5)
3.9
(2.0 to 16.1
a
)
HR (95% CI)
0.70 (0.53, 0.92)
6-month event-free rate — %
32.2
17.1
12-month event-free rate — %
14.9
6.2
Patients with ongoing
response — no. (%)
b
18 (14.9)
7 (5.4)
Clinical data cut-off date: April 24, 2018, 11 months after the last patient was enrolled. CI, confidence interval. HR, hazard ratio; CP/ET, carboplatin + etoposide.
Adapted from The New England Journal of Medicine, Horn L, et al, First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer, Epub ahead of








