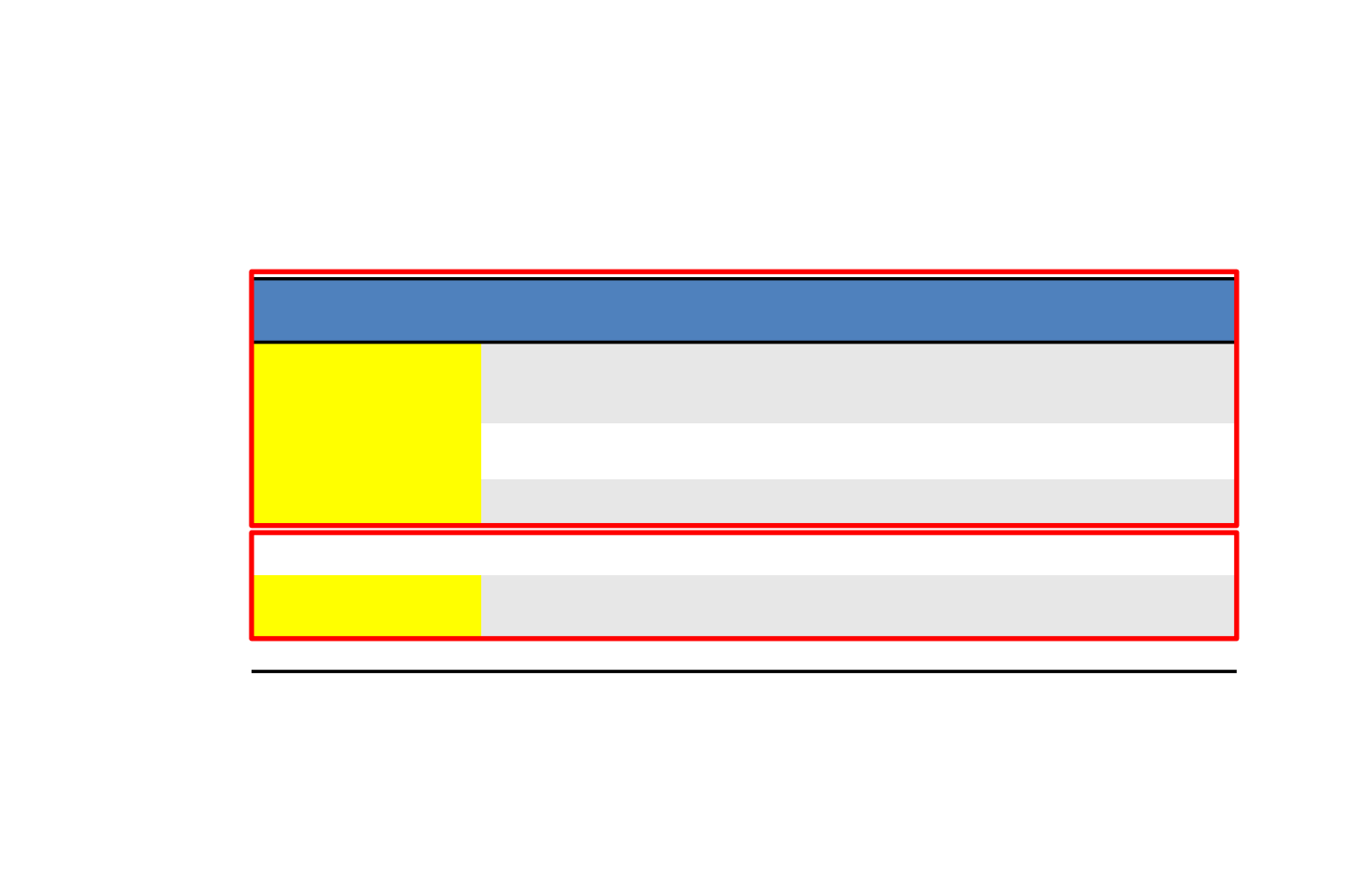
ALK TKI
Status
Indication
Ceritinib
FDA/EMA Approved
Naïve (2016)
After crizo (2014)
Alectinib
FDA/EMA Approved
Naïve (2017)
After crizo (2015)
Brigatinib
FDA Approved
After crizo (2017)
Ensartinib
Investigational
Lorlatinib
FDA Breakthrough
Therapy Designation
After crizo (2018)
TPX-0005
Investigational
Next Generation ALK inhibitors
2
nd
gen
3
rd
gen








