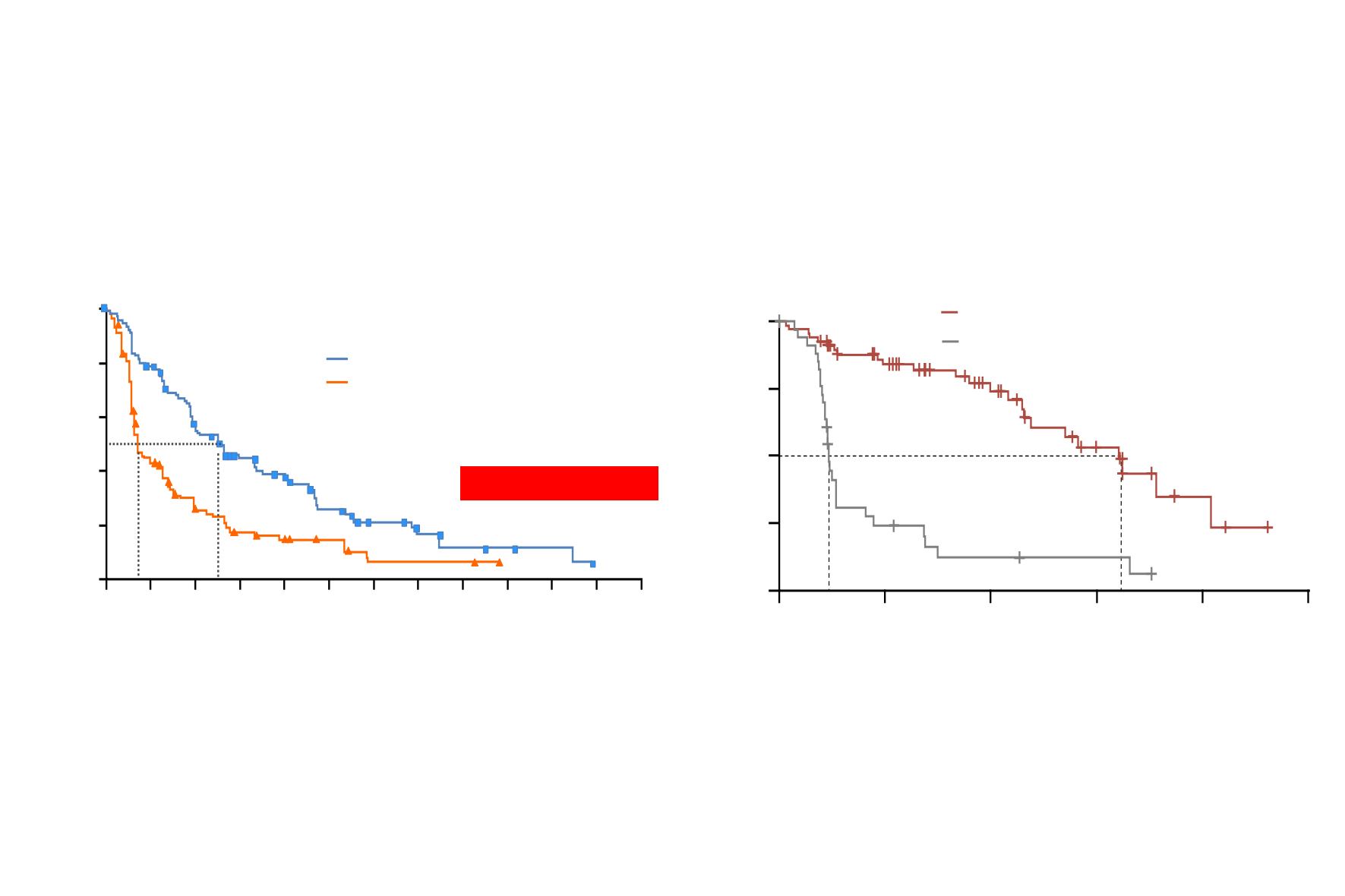
ASCEND-5 phase 3 study:
Primary Endpoint PFS by BIRC
Scagliotti et al. ESMO 2016; Ann Oncol 2016; Shawn Lancet Oncol 2017
Probability of PFS (BIRC) %
Time, months
80
60
40
20
0
0
4
8
12
16
20
24
100
Treatment
Median PFS,
mo (95%CI)
Events,
n (%)
Ceritinib 750 mg (n=115) 5.4 (4.1, 6.9) 83 (72.2)
Chemotherapy (n=116)
1.6 (1.4, 2.8) 89 (76.7)
HR 0.49 (95%CI 0.36, 0.67), p<0.001
2
6
10
14
18
22
5.4
months
1.6
months
RR by BIRC 39.1% vs 6.9%
5.4 mo
ALUR phase 3 study:
Primary Endpoint PFS Investigator-assessed
HR=0.15 [95% CI: 0.08–0.29]; p<0.001
Alectinib*
Median 9.6 months [95% CI: 6.9–12.2]
Chemotherapy
‡
Median 1.4 months [95% CI: 1.3–1.6]
1.00
0.75
0.50
0.25
0.00
0
3
6
9
12
15
Time (months)
PFS probability
1.4 mo
9.6 mo
Novello, ESMO 2017
* Cut-off median follow-up 6.5 months alectinib and 5.8 months chemotherapy








