Scagliotti et al. ESMO 2016; Ann Oncol 2016
Shaw A et al. Lancet Oncol 2017
Novello,et al
Annals of Oncology 29: 1409–1416, 2018
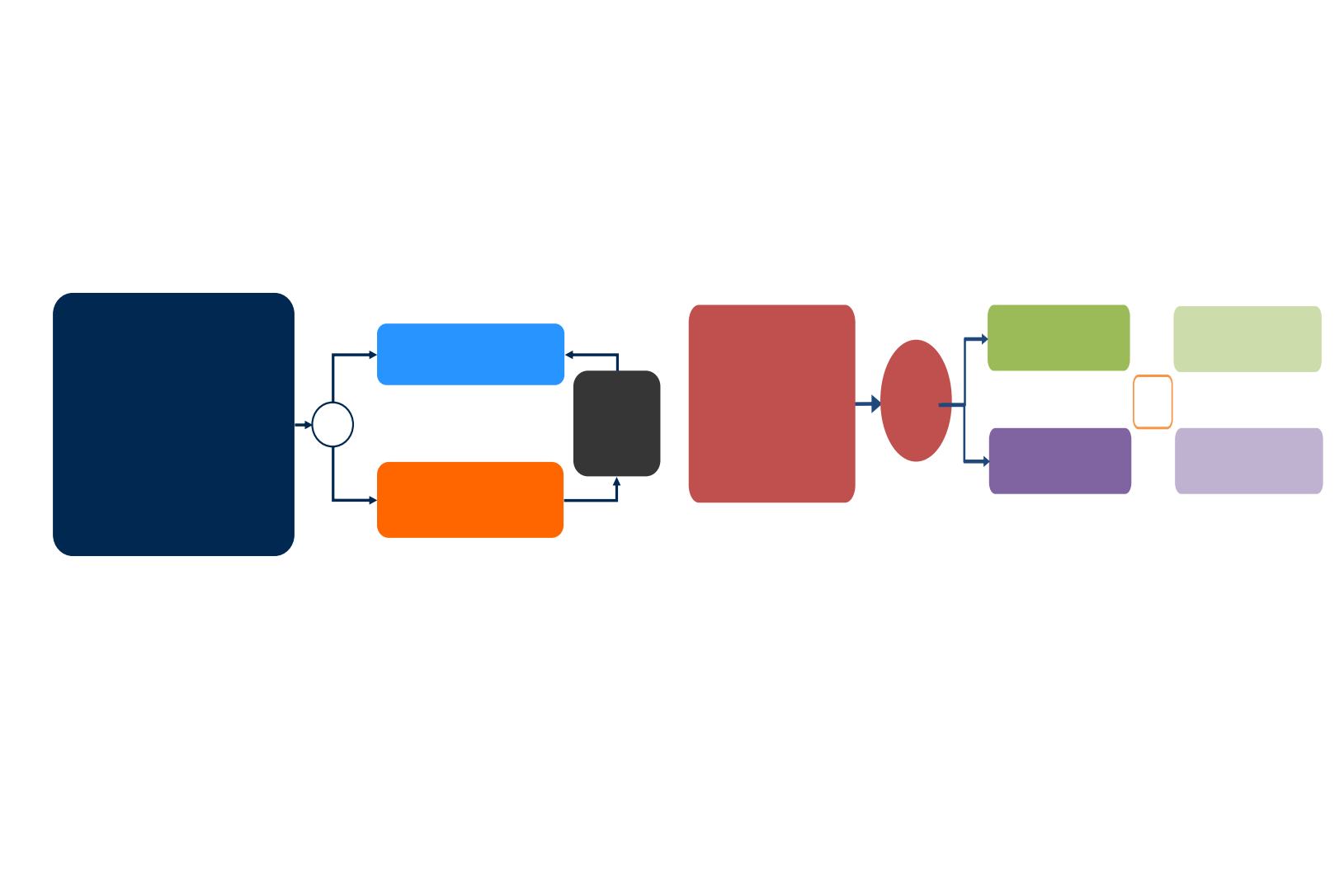
ASCEND-5 phase 3 study:
Second line Ceritinib
vs
Chemotherapy
R
1:1
Crossover
following
BIRC-
confirmed
PD
Stratification
• WHO PS (0 vs.1–2)
• Brain metastases (yes vs. no)
Key patient inclusion criteria
• Locally advanced or metastatic
ALK+ NSCLC
• Progressive disease
• WHO PS 0–2
• Prior crizotinib (>1 course
allowed)
• 1 or 2 prior chemotherapy
regimens
• Measurable disease at baseline
(n=231)
Chemotherapy
Pemetrexed 500 mg/m
2
(n=40) or docetaxel
75 mg/m
2
(n=73) q3w
Ceritinib 750 mg QD
PO
(n=115)
Primary endpoint:
PFS (BIRC)
Secondary endpoints:
OS, PFS (investigator), ORR, DCR, TTR
PD
Alectinib 600mg BID
Pemetrexed 500mg/m
2
q3w or docetaxel
75mg/m
2
q3w
Optional continuation of
alectinib if clinical benefit
R* 2:1
Crossover to
alectinib allowed
KEY ELIGIBILITY
●
Advanced or metastatic
ALK+
NSCLC
●
One prior line of platinum-
based chemotherapy
●
Crizotinib failure
●
ECOG PS 0−2
Primary endpoint
Investigator-assessed PFS in the ITT population
CNS ORR in patients with measurable CNS disease at baseline as assessed by an IRC (key
secondary endpoint)
; IRC-assessed PFS; systemic ORR; DCR and DOR; PFS in patients with CNS
metastases at baseline; time to CNS progression by baseline CNS disease status; CNS DCR and CNS
DOR in patients with CNS metastases at baseline; OS; safety
ALUR phase 3 study:
Second line Alectinib
vs
Chemotherapy
Primary endpoint
PFS Investigator-assessed
Secondary endpoints
CNS ORR by an IRC (key secondary endpoint); IRC-assessed PFS; systemic
ORR; DCR and DOR; PFS in patients with CNS metastases at baseline; time to
CNS progression by baseline CNS disease status; CNS DCR and CNS DOR in
patients with CNS metastas at aseline; OS; safety








