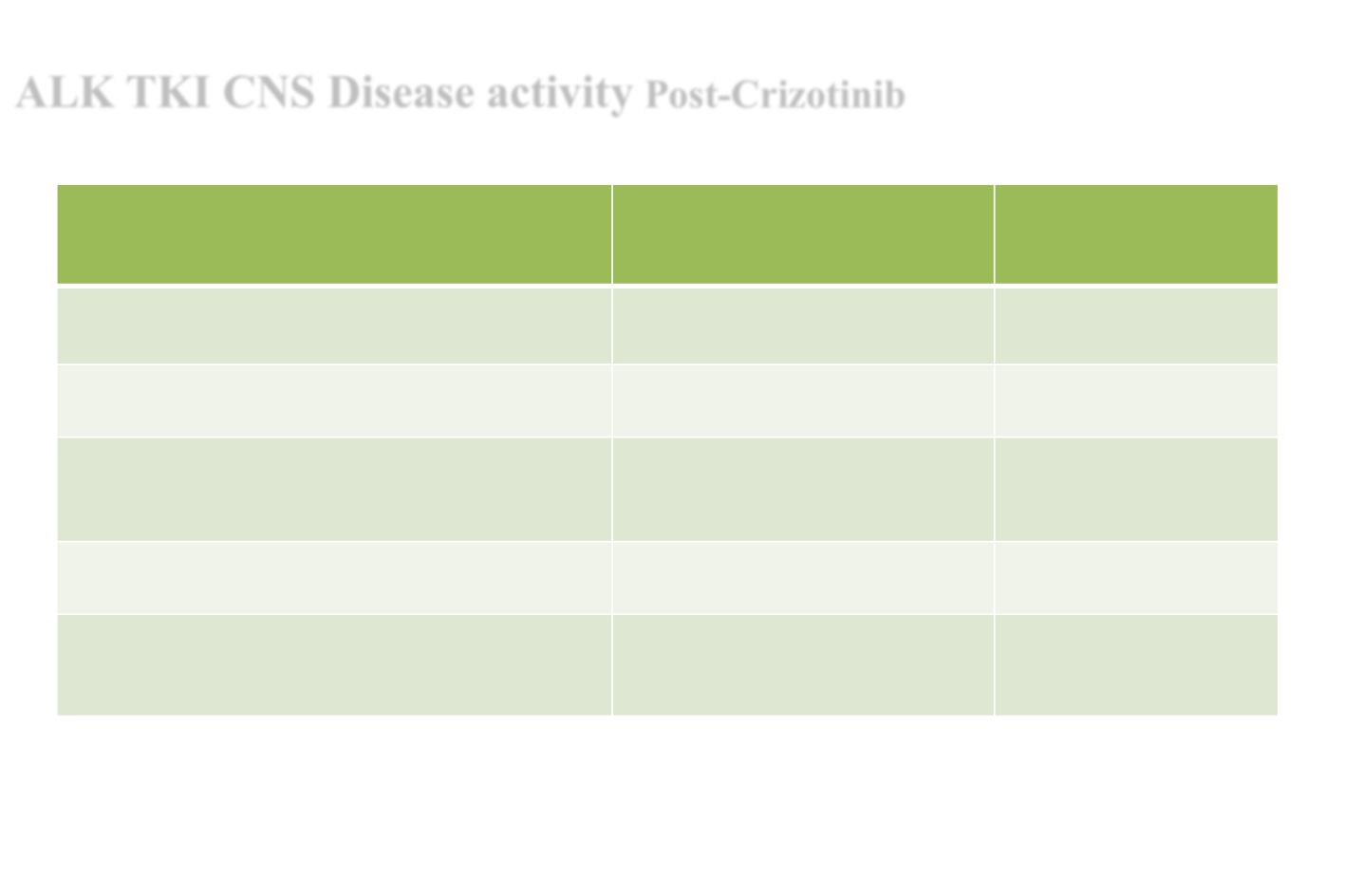
ALK TKI CNS Disease activity
Post-Crizotinib
Drug/ TRIAL
Objective response rate in CNS
measurable disease
CNS PFS
Ceritinib
ASCEND 5 Phase III
35%
mPFS 4.4m
Alectinib
ALUR Phase III
54%
mPFS 9.6
Brigatinib
ALTA Phase II
90->180mg dose
67%
mPFS 18.4 m
Lorlatinib
Phase II (Multiple prior TKIs permissible)
48-68%
NR
Ensartinib
64.3%
NR
Shaw A, et al. Lancet Oncol 2017
De Castro J, et al. ESMO 2017
Ahn W, et al. WCLC 2017
Solomon et al, WCLC 2017
Horn L, et al. Clin Cancer Res 2018








