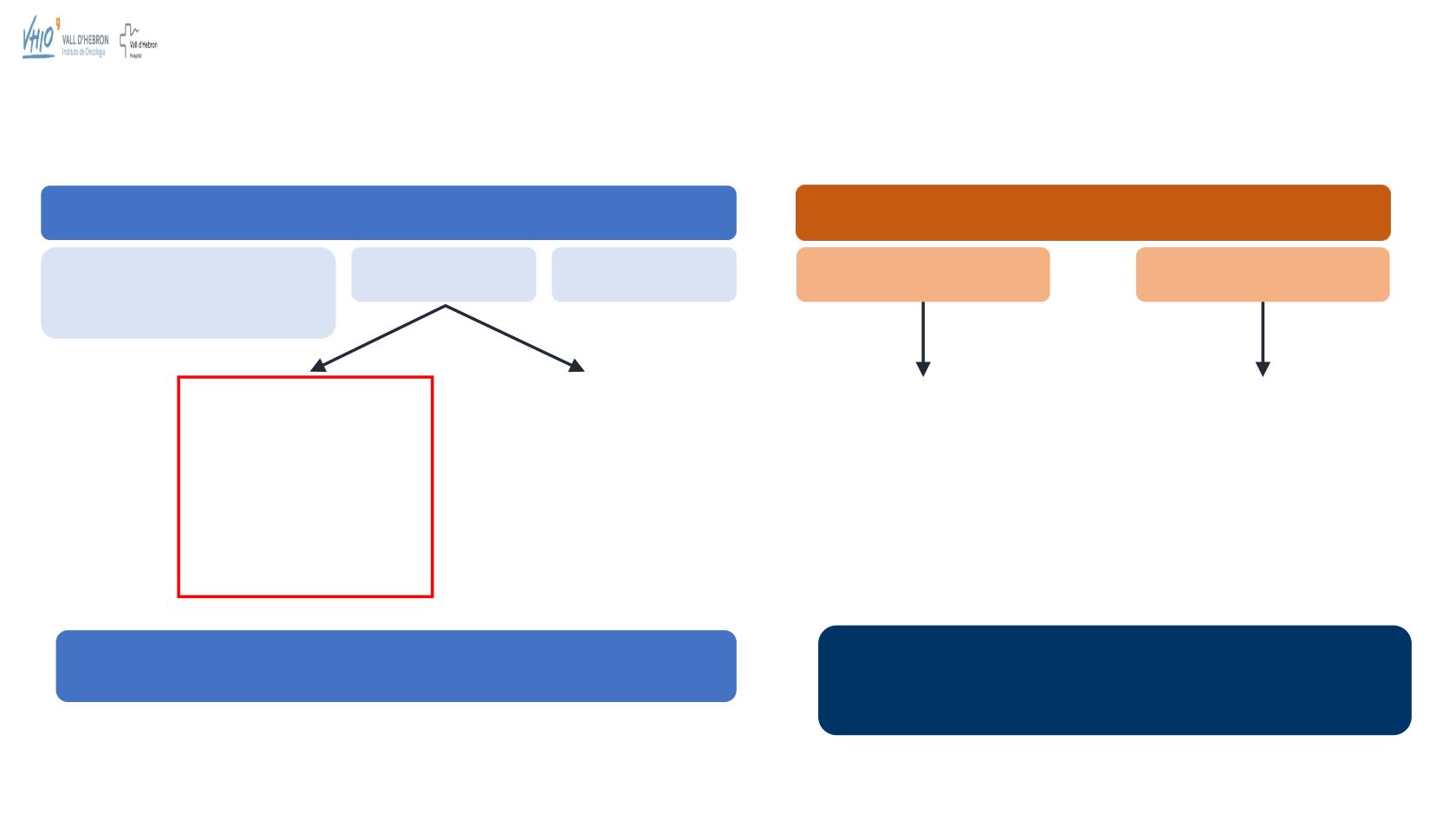
Study 10 and ARIEL2:
Studies Design
Relapsed ovarian cancer,
all comers
≥1 prior platinum-based
treatment
Platinum-sensitive
Study 10
CO-338-010
ARIEL2
CO-338-017
Part 1
Phase I
Dose escalation
Any solid tumour, including lymphoma
Part 2
Phase II
Part 3
Phase II/PK
Part 1
Phase II
Part 2
(Phase II extension)
Part 2A
Relapsed ovarian cancer
(g
BRCA
mut)
2–4 prior chemotherapy
treatments (last
treatment=platinum)
Platinum-sensitive
Part 2B
Relapsed ovarian cancer
(g
BRCA
mut or s
BRCA
mut)
3–4 prior chemotherapy
treatments
6-month treatment-free
interval after first regimen
Any platinum status
Relapsed high-grade
ovarian cancer
3–4 prior chemotherapies
Treatment-free interval
of >6 months following
first-line chemotherapy
Any platinum status
• Data from patients in Part 2A only are assessed in the EFFICACY analysis
• Data from patients in Parts 1, 2A and 3 are assessed in the SAFETY analysis
• Data from
mutBRCA population
receiving ≥2 prior lines of
chemotherapy are included in the EFFICACY analysis
• All enrolled patients receiving ≥1 dose of rucaparib are included in
the SAFETY analysis
Oza AM et al.
Gynecol Oncol
. 2017;147:267–275








