6
Platinum sensitive
relapse
2
nd
Platinum based* or
non-platinum therapy
Randomisation
2:1
>6 months/
183 days in
response
Monotherapy:
Oral Olaparib tablet
(4 tablets/day)
300mg/bd
Investigators
choice Comparator
(Weekly
Paclitaxel; Topotecan; Pegylated
Liposomal Doxorubicin; Gemcitabine
STRATIFICATION
Ø
Chemotherapy regimen choice
Ø
2 or 3 vs 4+ prior chemotherapy
Ø
Time to disease progression from end of last
platinum treatment
•
gBRCA mutation
•
High grade serous ovarian cancer or high
grade endometrioid cancer
•
At least 2 previous platinum therapy
•
Platinum Sensitive Relapase
Platinum based
therapy
*
Patient relapsed
>
6 months /183 days
in response if last
therapy platinum
based
Patient Screening
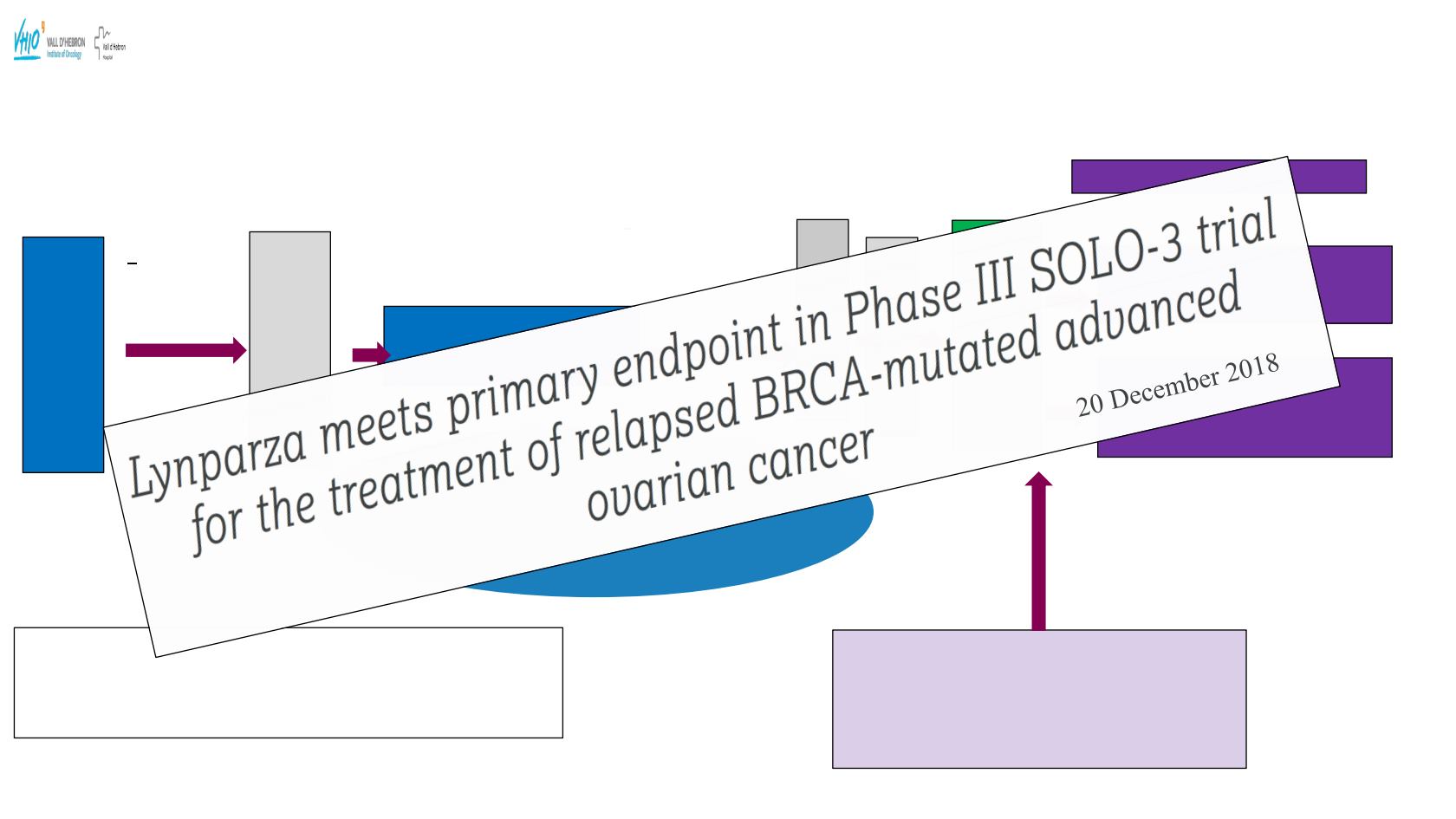
SOLO3:
A Phase III, Open Label, Randomised, Controlled, Multi-centre Study to assess the efficacy and safety of
Olaparib Monotherapy versus Physician’s Choice Single Agent Chemotherapy in the Treatment of Platinum
Sensitive Relapsed Ovarian Cancer in Patients carrying germline BRCA1/2 Mutations
Sample Size: 250
1º End-Point: ORR
in pts with measurable disease by BIDC
2º End-Point: PFS
ClinicalTrials.gov Identifier:NCT02282020








