Olaparib Monotherapy in g
BRCA
-Mutated Ovarian Cancer
N=137
Objective response rate (95% CI)
34% (26–42)
Complete response
2%
Partial response
32%
Median DoR in months (95% CI)
7.9 (5.6–9.6)
Objective response and DoR in patients receiving
three or more prior lines of chemotherapy
2
19 December 2014:
US FDA licensed
olaparib
for
monotherapy in patients with deleterious or suspected
deleterious
germline BRCA-mutated
(as detected by an FDA-
approved test) advanced ovarian cancer who have been
treated with ≥3 prior lines of chemotherapy
3
1.Kaufman B et al.
J Clin Oncol.
2014;33:244–250;
2. Domchek SM et al.
Gynecol Oncol
. 2016;140:199–203
3. AstraZeneca. LYNPARZA Prescribing Information. 2014
q
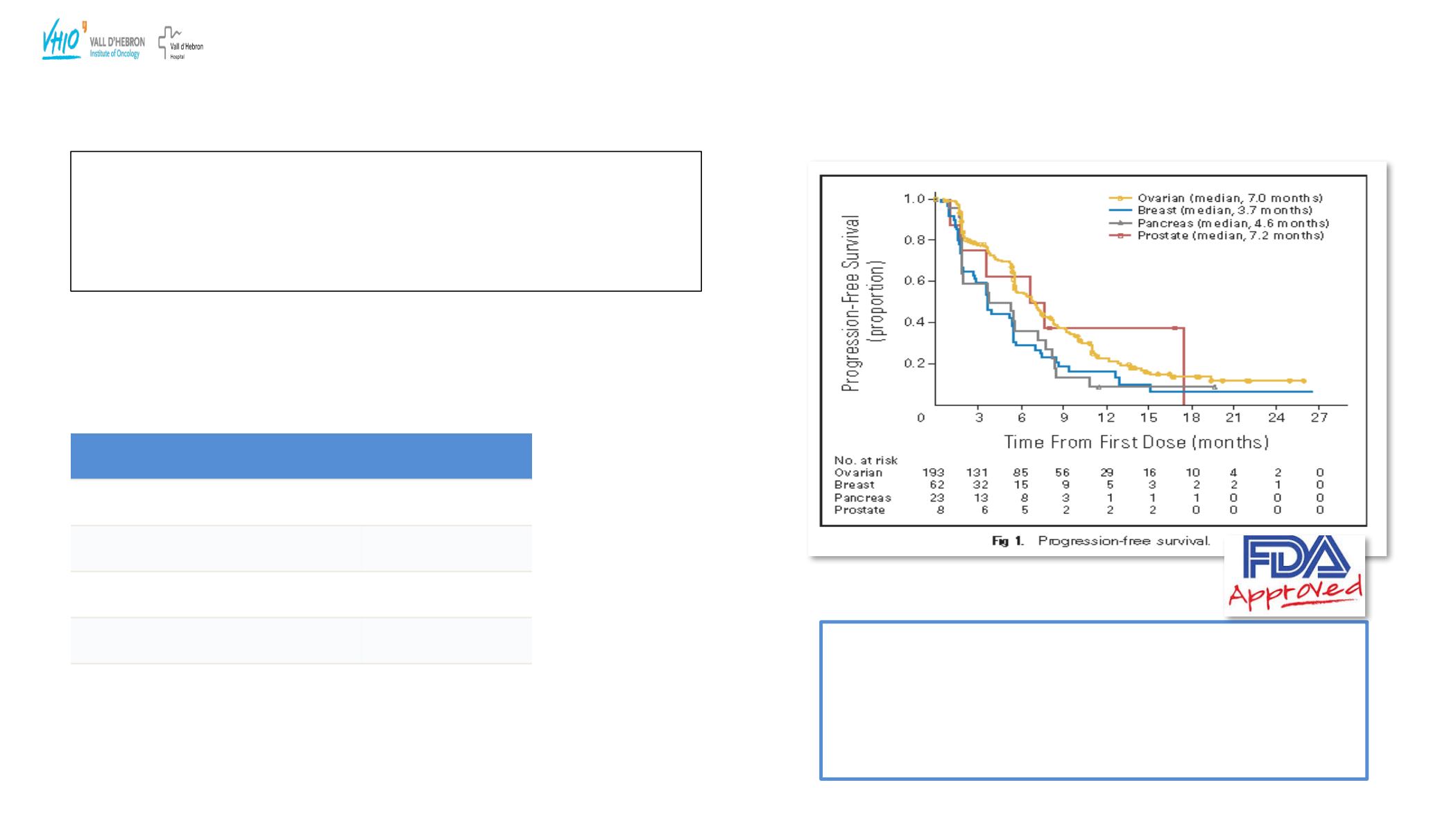
Ovarian cancer cohort( OvCa+ FT+ PP) :
193/298 patients (65%
of the overall study population)
•
All pts had received prior platinum chemotherapy.
•
Eligibility criteria: Platinum Resistant or ”not suitable for further platinum therapy”
( toxicity or hypersensitive reactions)
•
77% gBRCA 1
•
23% gBRCA 2
•
Mean previous lines of QT:4.4( 1-14)








