PARP inhibitors as maintenance therapy:
Where do we stand in sensitive relapsed ovarian cancer by Regulatory Agencies ?
ZEJULA™ [package insert]. Waltham, MA: TESARO, Inc.; 2017; ZEJULA™ [product information] London, UK: TESARO, UK Ltd.; 2017 LynparzaTM [package insert].
Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2017; LynparzaTM [product information]. Cheshire, UK: AstraZeneca UK Limited; 2017; Rubraca
®
(rucaparib) [package
insert]. Boulder, CO: Clovis Oncology, Inc: 2017; “Clovis Oncology’s rucaparib significantly improved progression-free survival in all ovarian cancer patient Populations
Studied in Phase 3 ARIEL3 maintenance treatment trial”; news release, 2017.
Available at:
http://phx.corporate-ir.net/phoenix.zhtml?c=247187&p=irol-newsArticle&ID=2281511.Accessed 7 August 2017
1. Mirza MR, et al.
N Engl J Med
2016;375:2154–64; 2. Ledermann J, et al.
Lancet Oncol
2014;15:852–61
3. Pujade-Lauraine E, et al.
Lancet Oncol
2017;18:1274–84; 4. Coleman RL, et al.
Lancet
2017;390:1949‒61
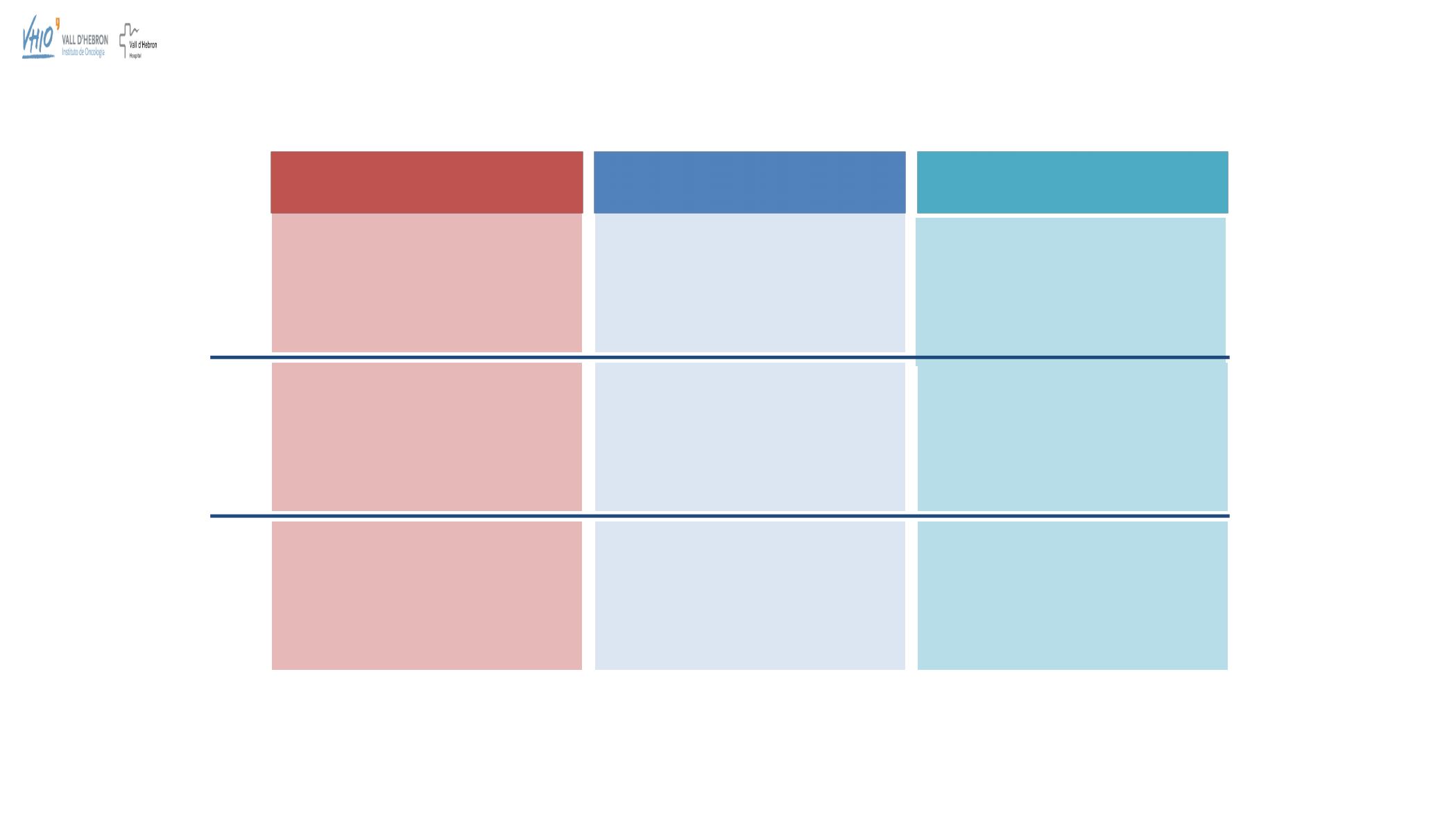
United
States
Europe
Maintenance treatment
of adult
patients with recurrent epithelial
ovarian, fallopian tube or primary
peritoneal cancer
regardless of
BRCA
status
Maintenance treatment
of adult
patients with recurrent epithelial
ovarian, fallopian tube or primary
peritoneal cancer
regardless of
BRCA
status
Maintenance treatment
of adult
patients with recurrent epithelial
ovarian, fallopian tube or primary
peritoneal cancer
regardless of
BRCA
status
Maintenance treatment
of adult
patients with
high-grade serous
epithelial ovarian, fallopian tube or
primary peritoneal cancer
regardless of
BRCA
status
Maintenance treatment
of adult
patients with recurrent epithelial
ovarian, fallopian tube or primary
peritoneal cancer
regardless of
BRCA
status
Maintenance treatment
of adult
patients with recurrent epithelial
ovarian, fallopian tube or primary
peritoneal cancer
regardless of
BRCA
status
ENGOT-OV16 / NOVA
1
Study 19
2
SOLO-2
3
ARIEL3
4
Key
trials
Niraparib
Olaparib
Rucaparib








