Integrated Efficacy Results:
2º End-Points: Duration of Response( DOR) and Progression Free Survival( PFS)
§
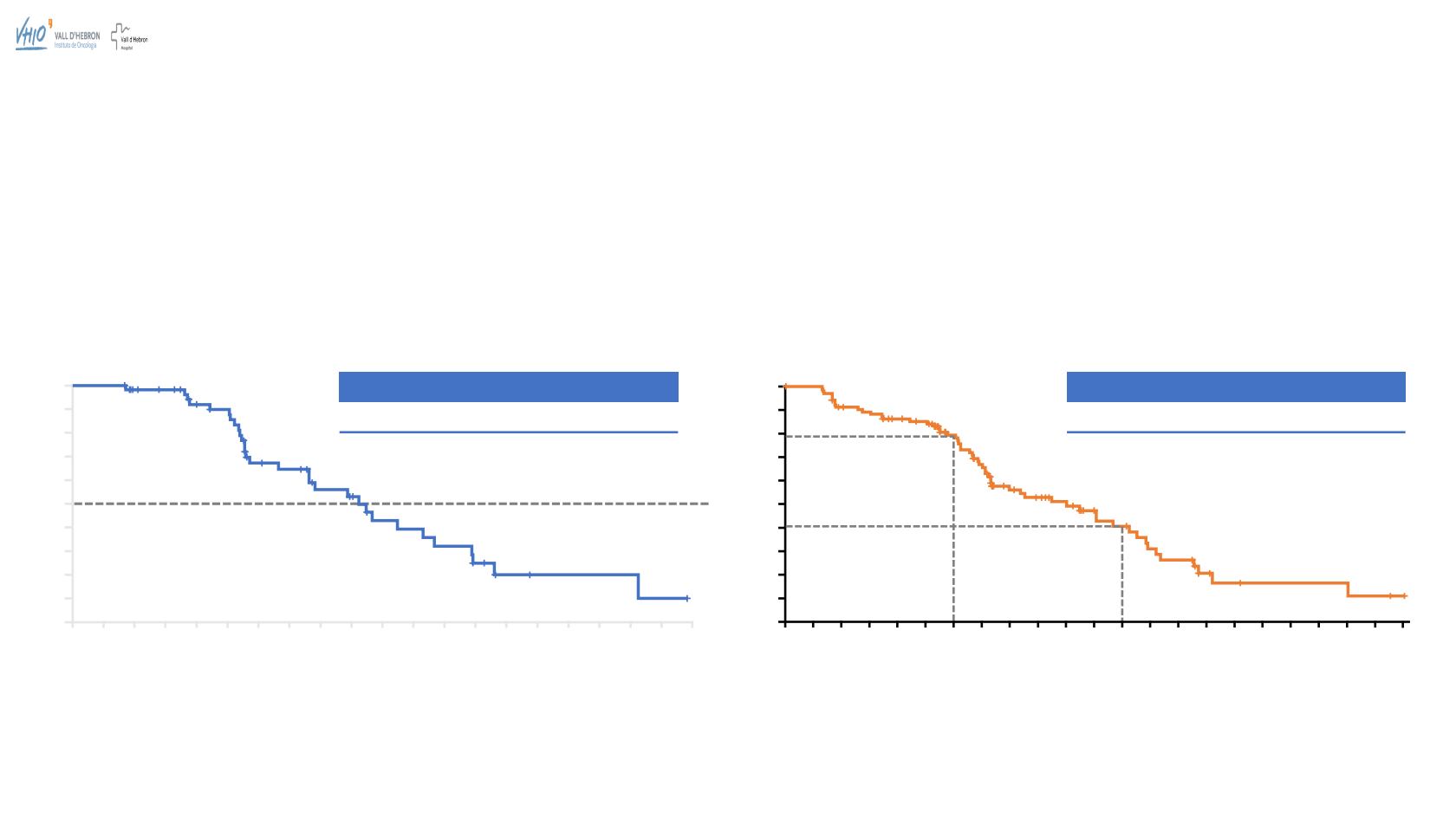
The median duration of investigator-assessed confirmed response was 9.2
months (95% CI 6.6–11.6)
§
The median DOR according to independent radiology review was
6.7 months (range 1.7–13.3; 95% CI 5.5–11.1 months)
Months, median 95% CI
Range
9.2
6.6–11.6 1.7–19.8+
Months
Probability of sustained response
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20
+
Censored; censoring rate: 47%
At risk (events)
57
(0)
57
(0)
52
(1)
50
(1)
44
(4)
41
(5)
27
(15)
25
(16)
19
(19)
17
(20) (23)
11
(24)
9
(26)
6
(28)
3
(29)
2
(29)
2
(29)
2
(29)
2
(29)
1
(30)
0
(30)
12
Oza AM et al.
Gynecol Oncol
. 2017;147:267–275
Integrated Efficacy Results: DOR
§
Investigator-assessed PFS was 10.0 months
(range 0.0–22.1; 95% CI 7.3–12.5)
§
47.2% had not progressed at the time of the visit cut-off
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22
Months
Probability of
progression-free survival
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
Progression-free at 6 months:
79%
Progression-free at 12 months:
41%
+
Censored; censoring rate: 47%
At risk (events)
106
(0)
85
(14)
69
(19)
43
(37)
31
(40)
21
(43)
14
(49)
8
(54)
3
(55)
3
(55)
2
(56)
0
(56)
93
(9)
Months, median 95% CI
Range
10.0
7.3–12.5 0.0–22.1+
Integrated Efficacy Results: PFS








