.
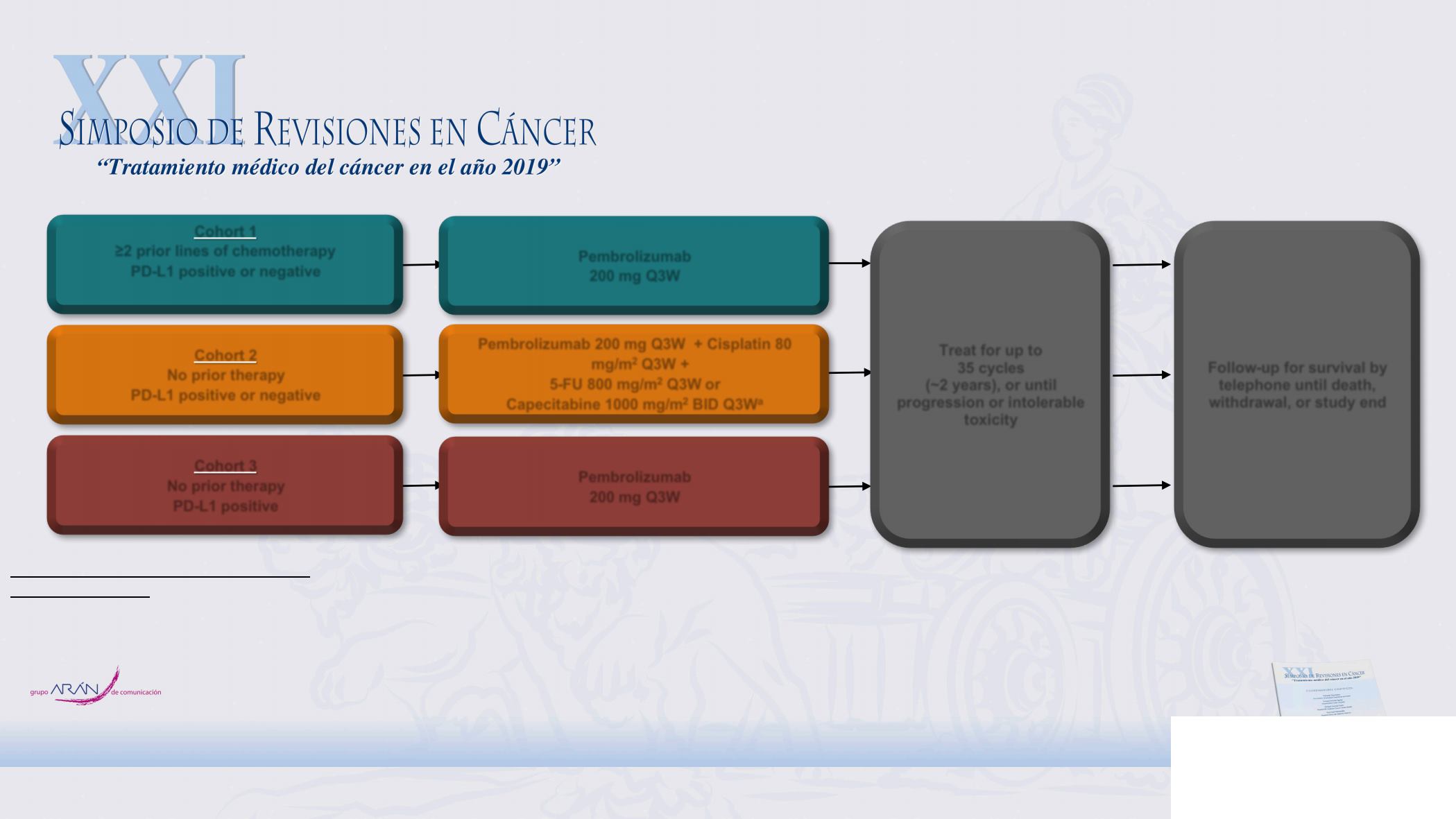
Cohort 3
No prior therapy
PD-L1 positive
Treat for up to
35 cycles
(~2 years), or until
progression or intolerable
toxicity
Follow-up for survival by
telephone until death,
withdrawal, or study end
Cohort 2
No prior therapy
PD-L1 positive or negative
Pembrolizumab 200 mg Q3W + Cisplatin 80
mg/m
2
Q3W +
5-FU 800 mg/m
2
Q3W or
Capecitabine 1000 mg/m
2
BID Q3W
a
Cohort 1
≥2 prior lines of chemotherapy
PD-L1 positive or negative
Pembrolizumab
200 mg Q3W
Pembrolizumab
200 mg Q3W
Response assessment per RECIST v1.1: First scan 9 weeks after cycle 1, then every 6 weeks for year 1 and every 9 weeks thereafter
Primary end points: Safety (all cohorts); ORR by central review per RECIST v1.1 (cohort 1: all patients and patients with PD-L1–positive expression); ORR by central review per RECIST v1.1
(cohort 3)
PD-L1 positive was defined as combined positive score (CPS) ≥1 (previously reported as and equivalent to CPS ≥1%), where
CPS = the number of PD-L1–positive cells
b
(tumor cells, lymphocytes, and macrophages) divided by the total number of tumor cells × 100
a
Capecitabine was administered only in Japan.
b
PD-L1 IHC 22C3 pharmDx (Agilent Technologies, Carpinteria, CA, USA).
KEYNOTE-059 (NCT02335411) Study Design
Kang ESMO GI 2017








