1
st
Line, PDL1+, Pembro monotherapy
a
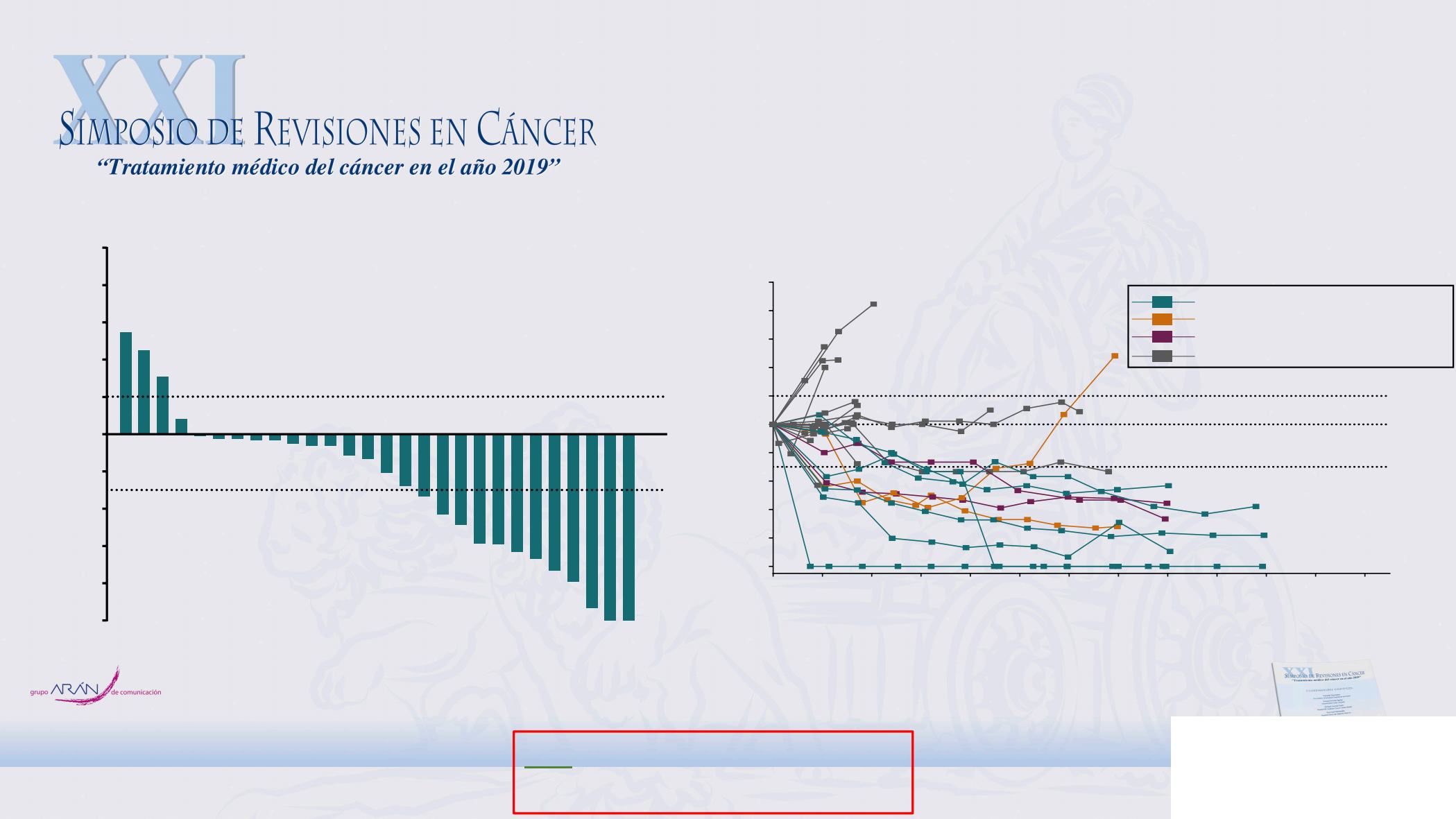
Only patients with measurable disease per RECIST v1.1 by central review at baseline who had ≥1 postbaseline assessment were included (n = 31) and assessments were nonevaluable/not available in 3
patients.
b
Longitudinal change in the sum of the longest target lesion diameters from baseline in patients with CR or PR (n = 30).
+No progressive disease at last disease assessment.
Data cutoff: April 21, 2017.
24 patients (77%) experienced
a reduction in target lesion size
Change From Baseline, %
30% decrease in tumor size
–100
–80
–60
–40
–20
0
20
40
60
80
100
Change From Baseline, %
20% increase in tumor size
0 2 4 6 8 10 12 14 16 18 20 22 24
–100
–80
–60
–40
–20
0
20
40
60
80
100
On treatment responder
Discontinued responder
On treatment nonresponder
Discontinued nonresponder
Median (range) duration of response: 9.6 (2.1-17.8+) months
Time Since Treatment Initiation, months
Best Percentage Change in All Patients (n = 31)
a
Longitudinal Change in All Patients (n = 30)
b
Kang ESMO GI 2017
ORR
25.8% (PD-L1 pos)








