Vemurafenib
Fase I (BRIM-1)→
Flaherty KT,
et al.
N Engl J Med 2010
Escalada de dosis
55 pacientes con tumores sólidos
• 49 melanoma metastásico
• 3 cáncer papilar tiroides
• 3 otros cánceres
Vemurafenib 160–1120 mg/12 horas p.o.
Objetivos
• Seguridad y fármacocinetica
• DMT
• Respuestas, SLP
960 mg/12 horas p.o. recomendada fase II
Fase extensión
32 pacientes melanoma metastásico
Mutación BRAF V600E
Objetivos
Respuestas
Toxicidad, fármacodinamia, PK
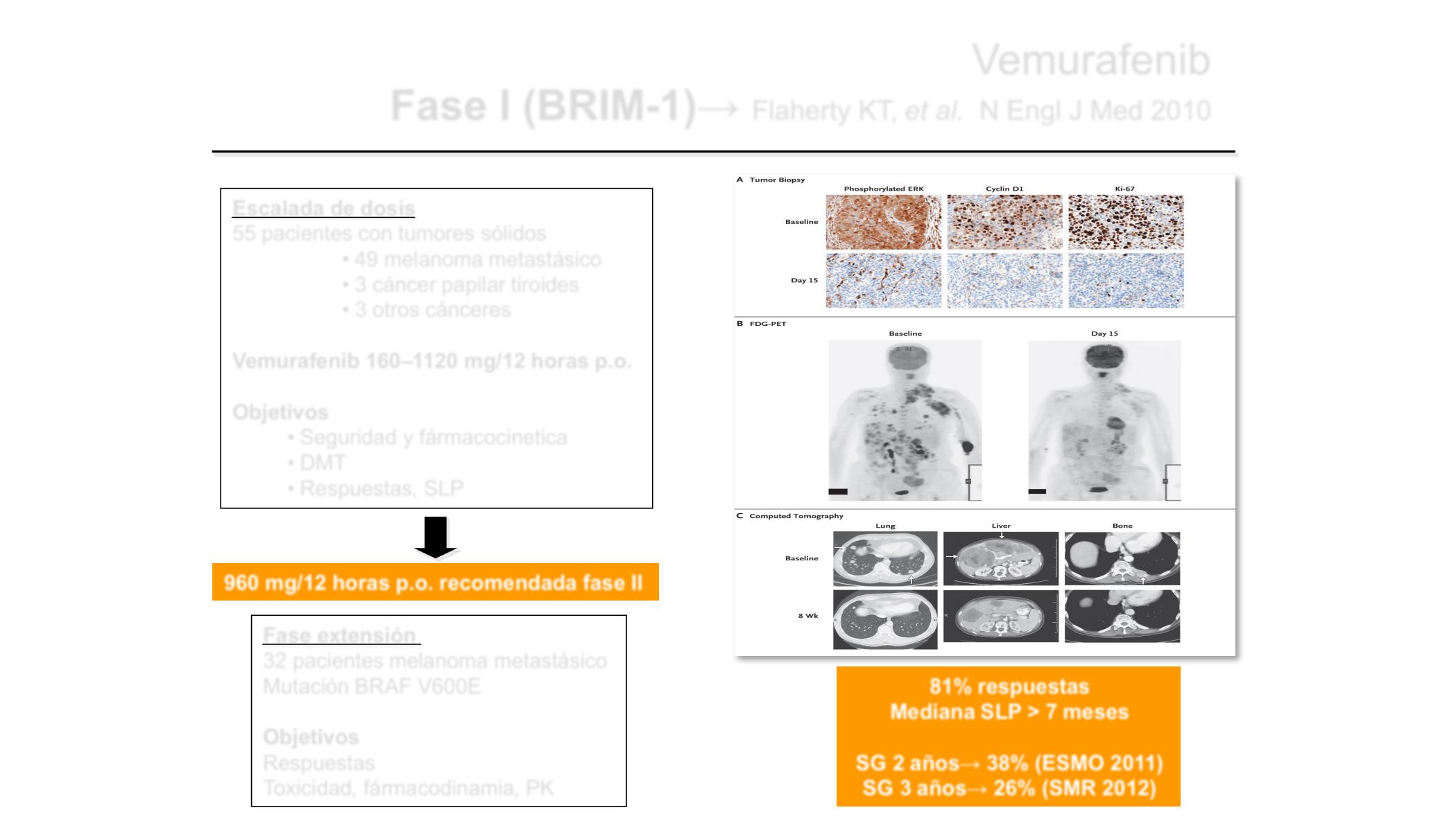
Figure 2. Representative Findings of the Effect of PLX4032 at the Recommended Phase 2 Dose
in Study Patients with Melanoma That Carried the V600E Mutation
The recommended phase 2 dose was 960 mg twice daily. Panel A (hematoxylin and eosin)
shows immunohisto-chemical analyses of the expression of phosphorylated extracellular
signal-regulated kinase (ERK), cyclin D1, and Ki-67 in tumor-biopsy specimens obtained at
baseline and on day 15 of treatment. Panel B shows the uptake of
18
F-fluorodeoxyglucose
(FDG) at baseline and on day 15 of treatment, as assessed by means of positron-emission
tomography (PET). Panel C shows computed tomographic images of lesions (arrows) in
lung, liver, and bone (with each pair of images shown for a different patient) at baseline and
at 8 weeks.
Flaherty et al.
Page 11
N Engl J Med
. Author manuscript; available in PMC 2013 July 26.
NIH-PA Author Manuscript
NIH-PA Author Manuscript
NIH-PA Author Manuscript
81% respuestas
Mediana SLP > 7 meses
SG 2 años→ 38% (ESMO 2011)
SG 3 años→ 26% (SMR 2012)








