ORIGINAL ARTICLE
Vemurafenib in patients with BRAF
V600
mutation-positive
metastatic melanoma: final overall survival results of the
randomized BRIM-3 study
P. B. Chapman
1
* , C. Robert
2
, J. Larkin
3
, J. B. Haanen
4
, A. Ribas
5
, D. Hogg
6
, O. Hamid
7
, P. A. Ascierto
8
,
A. Testori
9
, P. C. Lorigan
10
, R. Dummer
11
, J. A. Sosman
12
, K. T. Flaherty
13
, I. Chang
14
, S. Coleman
15
,
I. Caro
16
, A. Hauschild
17
& G. A. McArthur
18,19
1
Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, USA;
2
Department of Medicine, Institut Gustave Roussy and Paris Sud University,
Paris, France;
3
Department of Medicine, The Royal Marsden NHS Foundation Trust, London, UK;
4
Division of Medical Oncology, The Netherlands Cancer Institute,
Amsterdam, The Netherlands;
5
Department of Medicine, Hematology and Oncology, Jonsson Comprehensive Cancer Center at the University of California
Los Angeles, Los Angeles, USA;
6
Division of Medical Oncology and Hematology, Princess Margaret Hospital and University Health Network, Toronto, Canada;
7
The
Angeles Clinic and Research Institute, Melanoma Therapeutics, Los Angeles, USA;
8
Melanoma, Cancer Immunotherapy and Innovative Therapy Unit, Istituto
Nazionale Tumori Fondazione G. Pascale, Naples;
9
Melanoma and Sarcoma, Istituto Europeo di Oncologia, Milan, Italy;
10
Department of Medical Oncology,
University of Manchester, Manchester, UK;
11
Department of Dermatology, University of Zurich, Zurich, Switzerland;
12
Department of Hematology/Oncology, Robert
H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago;
13
Department of Medicine, Massachusetts General Hospital, Boston;
14
Department of
Biostatistics in Product Development, Biometrics;
15
Clinical Department;
16
Product Development, Oncology, Genentech Inc., South San Francisco, USA;
17
Department of Dermatology, University Hospital Schleswig-Holstein, Kiel, Germany;
18
Department of Oncology, Peter MacCallum Cancer Centre, East Melbourne;
19
Department of Oncology, University of Melbourne, Parkville, Australia
*
Correspondence to
: Dr Paul B. Chapman, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA. Tel:
þ
1-646-888-4162; E-mail:
chapmanp
@
mskcc.orgBackground:
The BRIM-3 trial showed improved progression-free survival (PFS) and overall survival (OS) for vemurafenib
compared with dacarbazine in treatment-naive patients with BRAF
V600
mutation–positive metastatic melanoma. We present
final OS data from BRIM-3.
Patients and methods:
Patients were randomly assigned in a 1 : 1 ratio to receive vemurafenib (960 mg twice daily)
or dacarbazine (1000 mg/m
2
every 3 weeks). OS and PFS were co-primary end points. OS was assessed in the intention-to-treat
population, with and without censoring of data for dacarbazine patients who crossed over to vemurafenib.
Results:
Between 4 January 2010 and 16 December 2010, a total of 675 patients were randomized to vemurafenib (
n
¼
337)
or dacarbazine (
n
¼
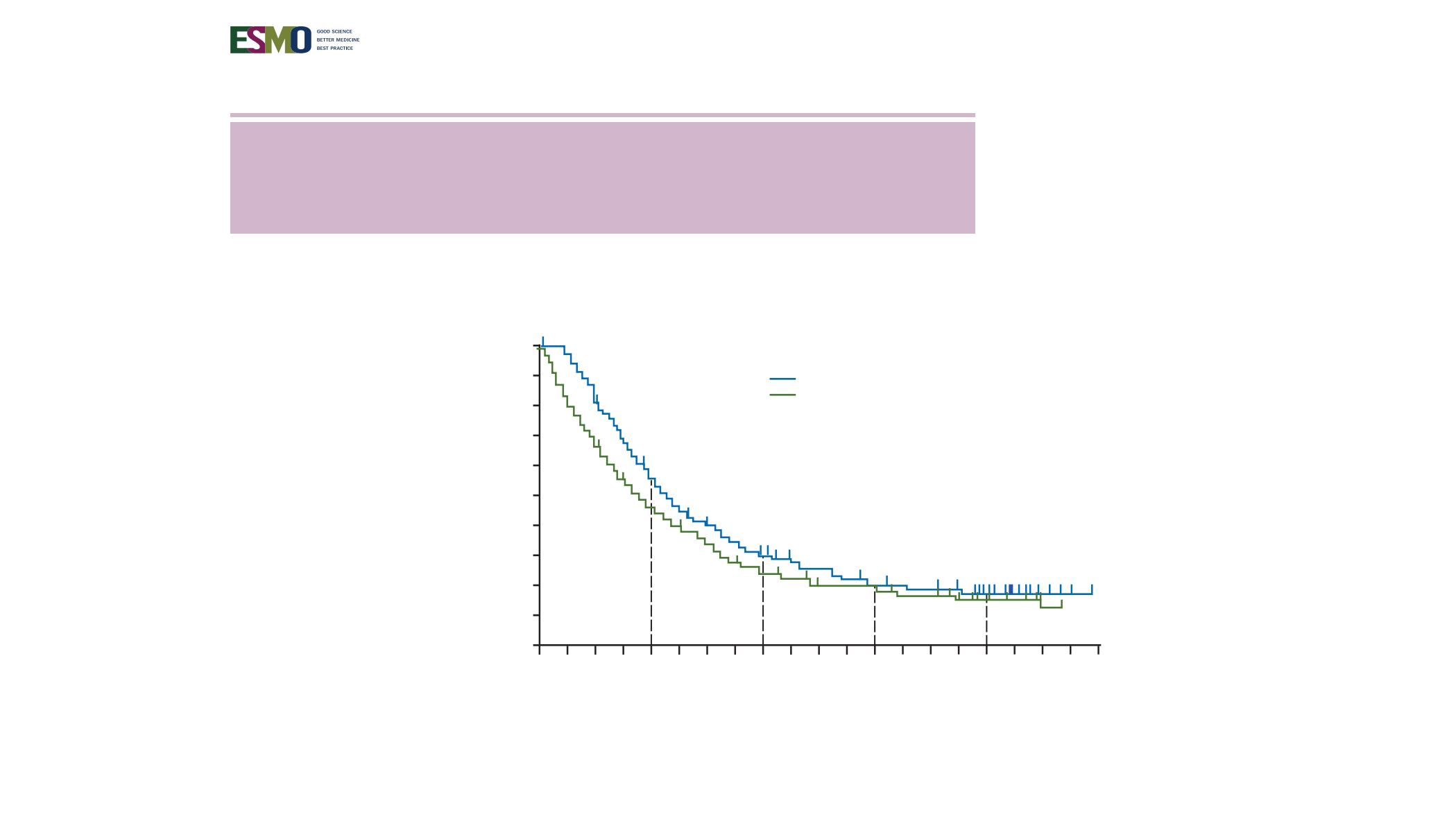
338, of whom 84 crossed over to vemurafenib). At the time of database lock (14 August 2015), median OS,
censored at crossover, was significantly longer for vemurafenib than for dacarbazine {13.6 months [95% confidence interval (CI)
12.0–15.4] versus 9.7 months [95% CI 7.9–12.8; hazard ratio (HR) 0.81 [95% CI 0.67–0.98];
P
¼
0.03}, as was median OS without
censoring at crossover [13.6 months (95% CI 12.0–15.4) versus 10.3months (95% CI 9.1–12.8); HR 0.81 (95% CI 0.68–0.96);
P
¼
0.01]. Kaplan–Meier estimates of OS rates for vemurafenib versus dacarbazine were 56% versus 46%, 30% versus 24%,
21% versus 19% and 17% versus 16% at 1, 2, 3 and 4 years, respectively. Overall, 173 of the 338 patients (51%) in the dacarbazine
arm and 175 of the 337 (52%) of those in the vemurafenib arm received subsequent anticancer therapies, most commonly
ipilimumab. Safety data were consistent with the primary analysis.
Conclusions:
Vemurafenib continues to be associated with improved median OS in the BRIM-3 trial after extended follow-up.
OS curves converged after
#
3 years, likely as a result of crossover from dacarbazine to vemurafenib and receipt of subsequent
anticancer therapies.
ClinicalTrials.gov:
NCT01006980.
Key words
:
melanoma, BRAF mutation, vemurafenib, dacarbazine
Annals of Oncology
28: 2581–2587, 2017
doi:10.1093/annonc/mdx339
Published online 2 August 2017
No. of patients at risk
Vem (
n
= 337)
DTIC (
n
= 338)
13.6 (12.0–15.4)
10.3 (9.1–12.8)
0.8 (0.7–1.0)
0.0142
Hazard ratio (95% CI)
P
-value
OS, median (95% CI), months
OS, %
100
80
60
40
20
0
Vem
DTIC
0
6
12
18
30
24
36
48
54
60
42
Time, months
3
9
15
37
21
33
45
51
57
39
337
338
281
210
183
140
130
103
78
58
96
73
63
53
40
28
13
2
0
0
56
45
326
254
323
171
149
117
86
65
111
83
69
57
52
40
25
18
2
0
61
47
90
70
50
30
10
55.7%
46.0%
30.2%
24.5%
20.8%
18.9%
17%
15.6%
Figure 2.
Kaplan–Meier curves for OS (without censoring at crossover) in the ITT population. CI, confidence interval; DTIC, dacarbazine;
Annals of Oncology
Original article








