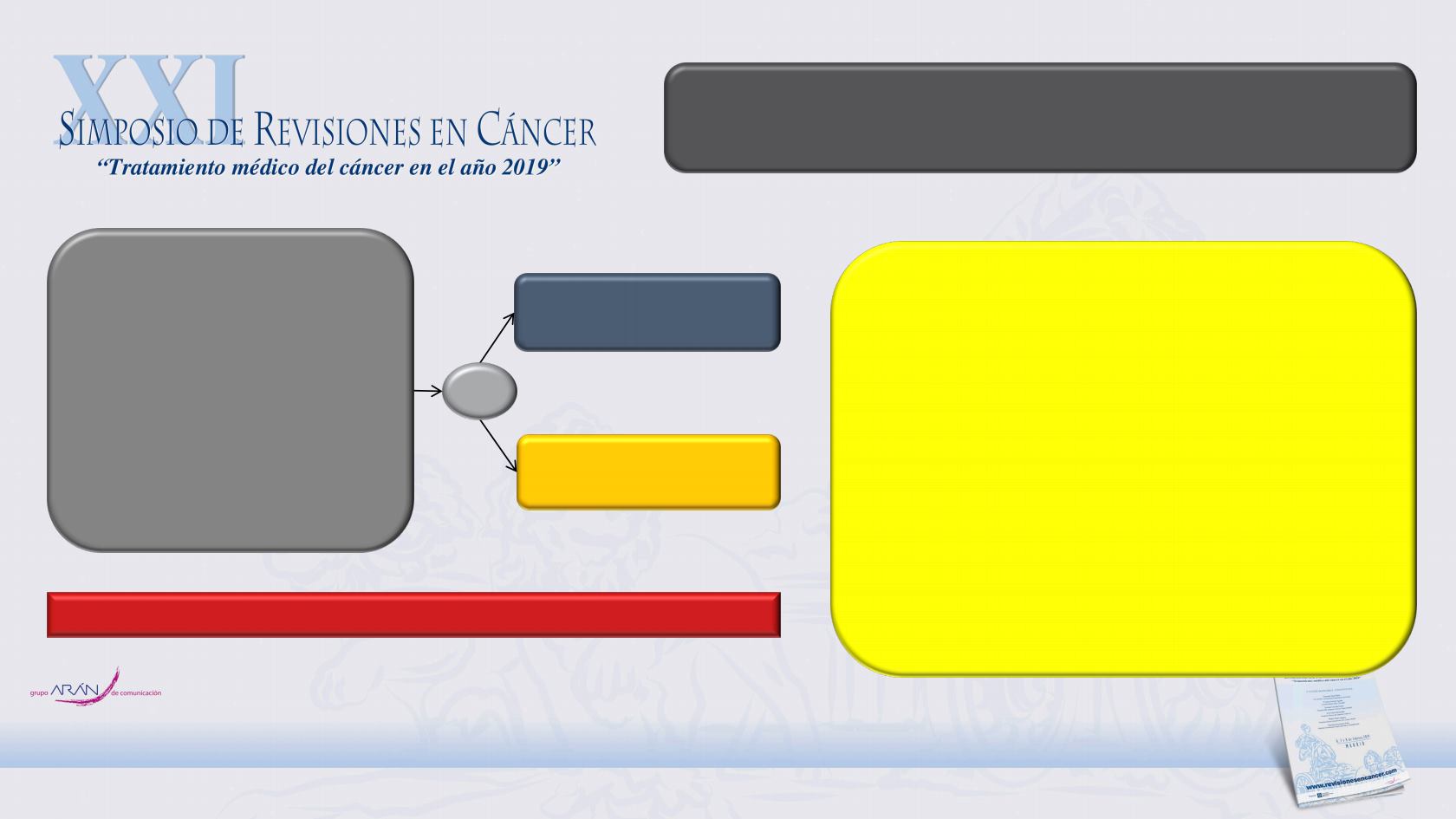
ClinicalTrials.gov: NCT01946204
Apalutamide
240 mg QD
Placebo
QD
R
2:1
N=1207
Non-metastatic (M0) CRPC
Testosterone
≤50 ng/dL
Progressive disease with
ongoing ADT
Asymptomatic
PSADT ≤10 months
•
SPARTAN is a multinational, phase 3, randomised, double-blind,
placebo-controlled trial
•
Primary endpoint: Metastasis-free survival
Primary endpoint: METASTASIS-FREE SURVIVAL BENEFIT
Secondary endpoints
•
Time to metastases
•
Progression-free survival (PFS)
•
Time to symptomatic progression
•
Overall survival
•
Time to first use of cytotoxic chemotherapy
Exploratory endpoints
•
PFS2
•
Time to PSA progression
•
PSA decline
•
Patient-reported outcomes (FACT-P, EQ-5D-3L)
Smith MR, et al. N Engl J Med 2018;378:1408-18








