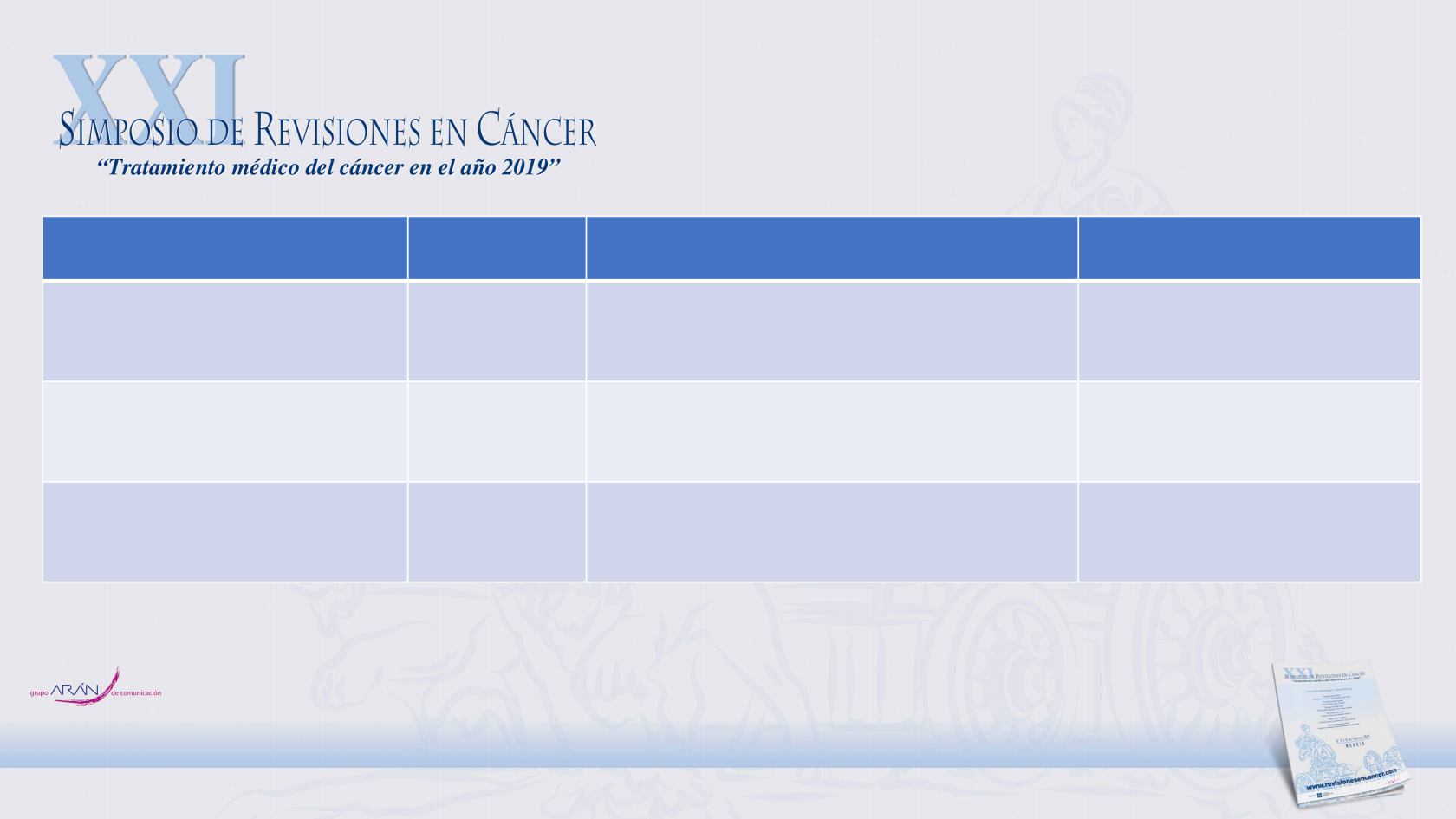
Trial
Enrollment
Treatment
Primary endpoint
PROSPER (NCT02003924)
1401
Enzalutamide 160 mg once daily vs
placebo
Metastasis-free survival
SPARTAN (NCT01946204)
1207
Apalutamide 240 mg once daily vs
placebo
Metastasis-free survival
ARAMIS (NCT02200614)
1509
Darolutamide 600 mg twice daily vs
placebo
Metastasis-free survival
Positive results from SPARTAN and PROSPER, both published at NEJM, 2018.
Positive results from ARAMIS (press release), communication at 2019 ASCO GU.
M0 CASTRATION-RESISTANT PROSTATE CANCER:
THE RIGHT PATIENT FOR CLINICAL TRIAL








