Smith MR, et al. N Engl J Med 2018;378:1408-18
SPARTAN – SECONDARY AND EXPLORATORY ENDPOINTS
the cause in 2 patients each) and in 1 patient in
the placebo group (with cardiorespiratory arrest
as the cause) (Table S5 in the Supplementary Ap-
pendix). The following adverse events that were
considered by the investigators to be related to the
vival was more than 2 years longer (40.5 months
vs. 16.2 months). The effect was observed across all
subgroups, including patients in all age groups,
those with short PSA doubling times, and those
with local or regional nodal disease at trial en-
try. Time to metastasis, progression-free surviv-
al, and time to symptomatic progression were
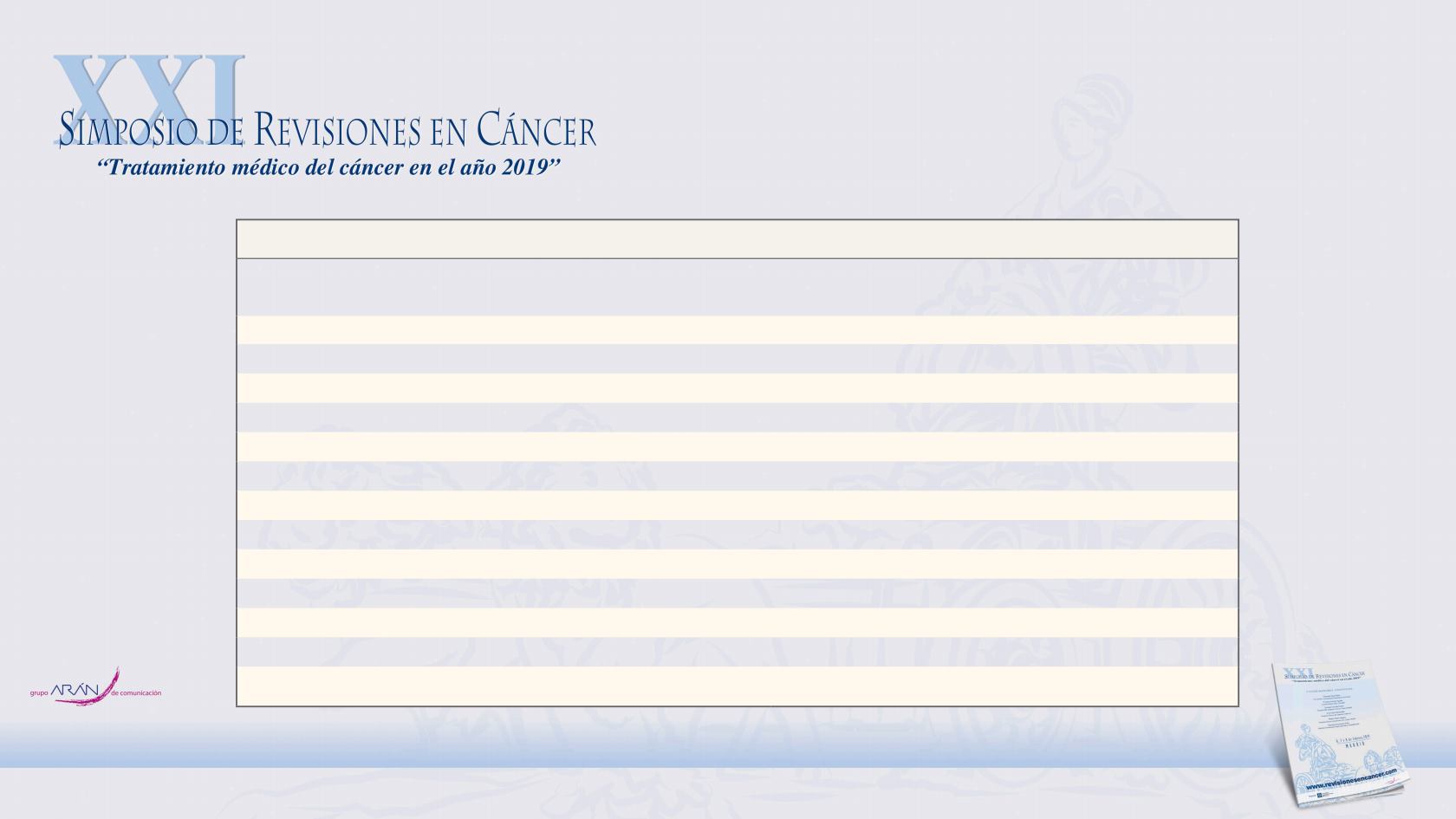
End Point
Apalutamide
(N=806)
Placebo
(N=401)
Hazard Ratio
(95% CI)
P Value
Secondary end points (mo)†
Median time to metastasis
40.5
16.6
0.27 (0.22–0.34)
<0.001
Median progression-free survival
40.5
14.7
0.29 (0.24–0.36)
<0.001
Median time to symptomatic progression
NR
NR
0.45 (0.32–0.63)
<0.001
Median overall survival
NR
39.0
0.70 (0.47–1.04)
0.07
Median time to the initiation of cytotoxic chemotherapy
NR
NR
0.44 (0.29–0.66)
—
Exploratory end points
Median second-progression–free survival (mo)
NR
39.0
0.49 (0.36–0.66)
Median time to PSA progression (mo)
NR
3.7
0.06 (0.05–0.08)
Patients with a PSA response (%)
89.7
2.2
40 (21–77)‡
Patient-reported outcomes§
Change in total FACT-P score from baseline to 29 months¶
−0.99±0.98 −3.29±1.97
—
Change in total EQ VAS score from baseline to 29 months∥
1.44±0.87
0.26±1.75
—
* Plus–minus values are means ±SE. NR denotes not reached, and PSA prostate-specific antigen.
† The P value for time to symptomatic progression crossed the O’Brien–Fleming efficacy boundary of 0.00008; the P value for overall survival
did not. The P value for time to the initiation of cytotoxic chemotherapy was not calculated because the P value for overall survival did not
cross the O’Brien–Fleming efficacy boundary.
‡ The comparison for this exploratory end point was calculated as a relative risk rather than a hazard ratio.
Table 2.
Prespecified Secondary and Exploratory End Points.*








