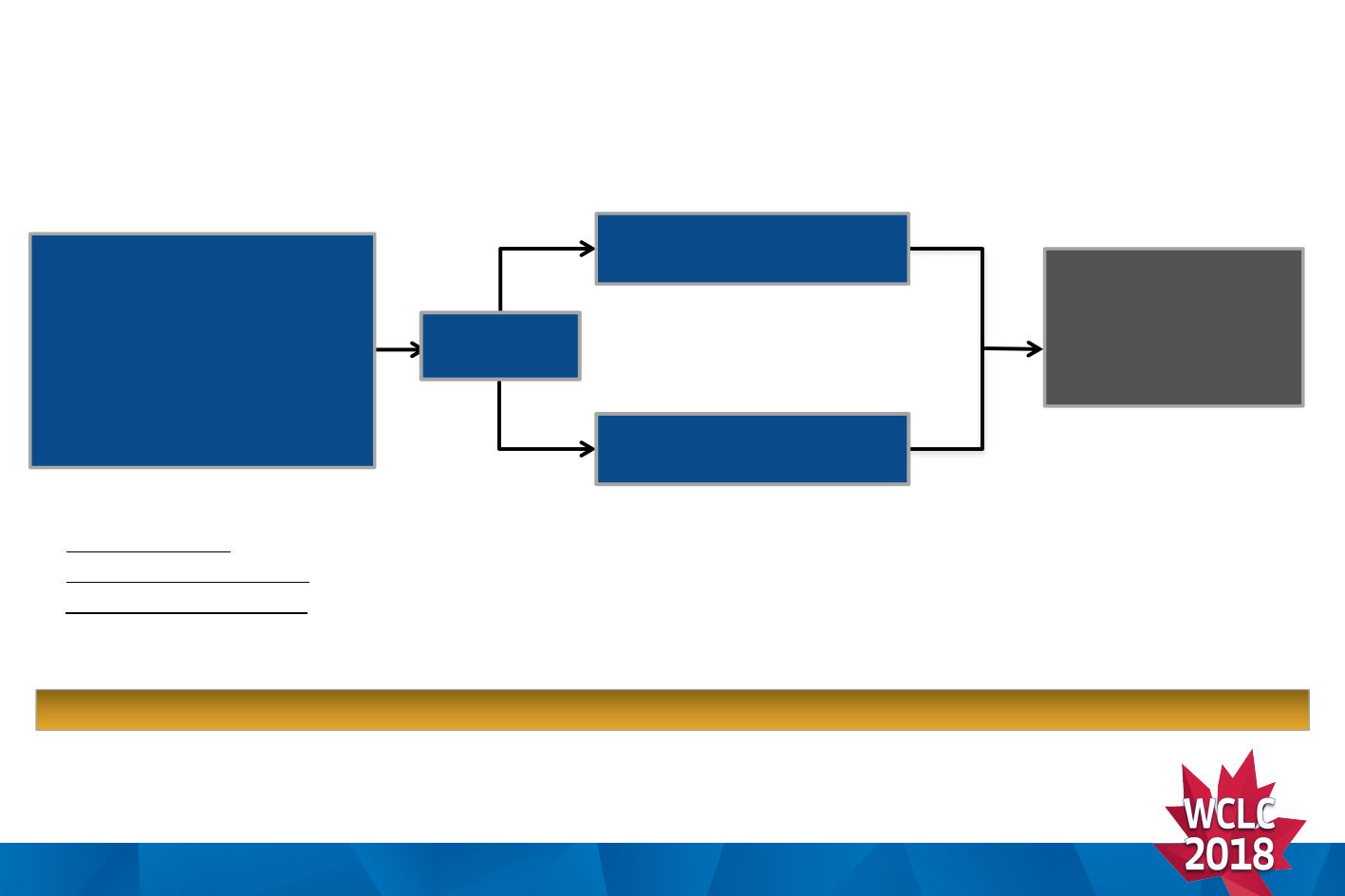
ALTA-1L: Phase 3, Open-label, Randomized, Multicenter, Study (NCT02737501)
•
Primary endpoint: Blinded independent review committee (BIRC)
–
assessed PFS per RECIST v1.1
•
Key secondary endpoints: Confirmed ORR, confirmed intracranial ORR, intracranial PFS, OS, safety, and tolerability
•
Statistical considerations: ~270 total patients (198 events); 135 in each arm to detect a 6-month improvement in PFS (HR=0.625),
assuming:
–
10-month PFS in crizotinib arm
–
2 planned interim analyses at 99 (50%) and 149 (75%) total expected events
First Interim Analysis:
•
A total of 99 PFS events are included
•
According to the pre-specified O’Brien Flemming Lan-DeMets alpha spending function, a 2-sided P-value of 0.0031 will be
used to define the threshold for significance
Stratified by:
•
Brain metastases at baseline (y/n)
•
Prior chemotherapy for locally
advanced or metastatic disease (y/n)
Randomized
1:1
Brigatinib 180 mg qd with
7-day lead-in at 90 mg
Crizotinib 250 mg bid
•
Stage IIIB/IV ALK+ NSCLC
‒
Enrollment based on local
ALK testing
•
No prior ALK inhibitor
•
≤1 prior systemic therapy for
locally advanced/metastatic
NSCLC
•
BIRC-assessed PD
*
•
Intolerable toxicity
•
Other reasons for
discontinuation
Trial fully accrued in August 2017 (N=275)
*Arm B crossover to
brigatinib permitted at
BIRC-assessed PD
Disease assessment every 8 weeks, including brain MRI for all patients
September 25, 2018, at
NEJM.org. DOI: 10.1056/NEJMoa1810171








