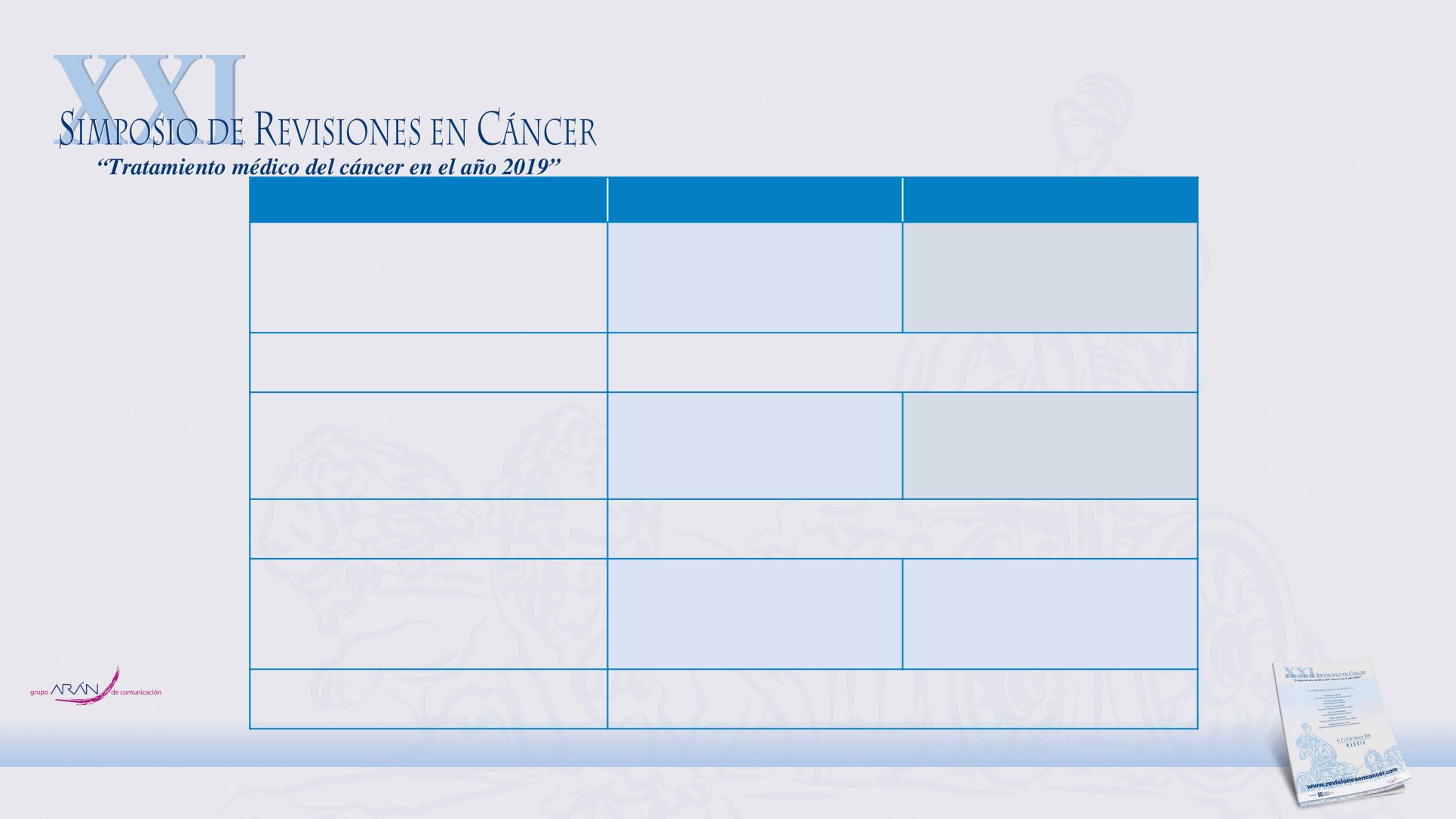
ESMO primary tumour location pooled analysis
Predictive analysis of tumour location (right vs left) in
trials comparing CTx + anti-EGFR with CTx ± bevacizumab*
Arnold D, et al. Ann Oncol 2017;28(8):1713-29
HR < 1 favours CTx + anti-EGFR; HR > 1 favours CTx ± bevacizumab;
OR > 1 favours CTx + anti-EGFR;
OR < 1 favours CTx ± bevacizumab.
No significant inter-study heterogeneity for any of the three endpoints.
†
Test comparing HR
Left
and HR
Right
/OR
Left
and OR
Right
.
WT
RAS
(pooled)
Total LEFT
Total RIGHT
OS
HR (95% CI), right vs left
P-value
0.75 (0.67‒0.84)
< 0.001
Favours CTx + anti-EGFR
1.12 (0.87‒1.45)
0.381
Favours CTx ± bevacizumab
HR
Interaction
(95% CI)
P-value HR
Interaction
†
1.50 (1.19‒1.88)
P < 0.001
PFS
HR (95% CI), right vs left
P-value
0.78 (0.70‒0.87)
< 0.001
Favours CTx + anti-EGFR
1.12 (0.87‒1.44)
0.365
Favours CTx ± bevacizumab
HR
Interaction
(95% CI)
P-value HR
Interaction
†
1.43 (1.14‒1.80)
P = 0.002
ORR
OR (95% CI), right vs left
P-value
2.12 (1.77‒2.55)
< 0.001
Favours CTx + anti-EGFR
1.47 (0.94‒2.29)
0.089
Favours CTx + anti-EGFR
OR
Interaction
(95% CI)
P-value OR
Interaction
†
0.69 (0.46‒1.04)
P = 0.07
*PRIME, Phase 2 PEAK, 181, FIRE-3, CRYSTAL, CALGB 80405








