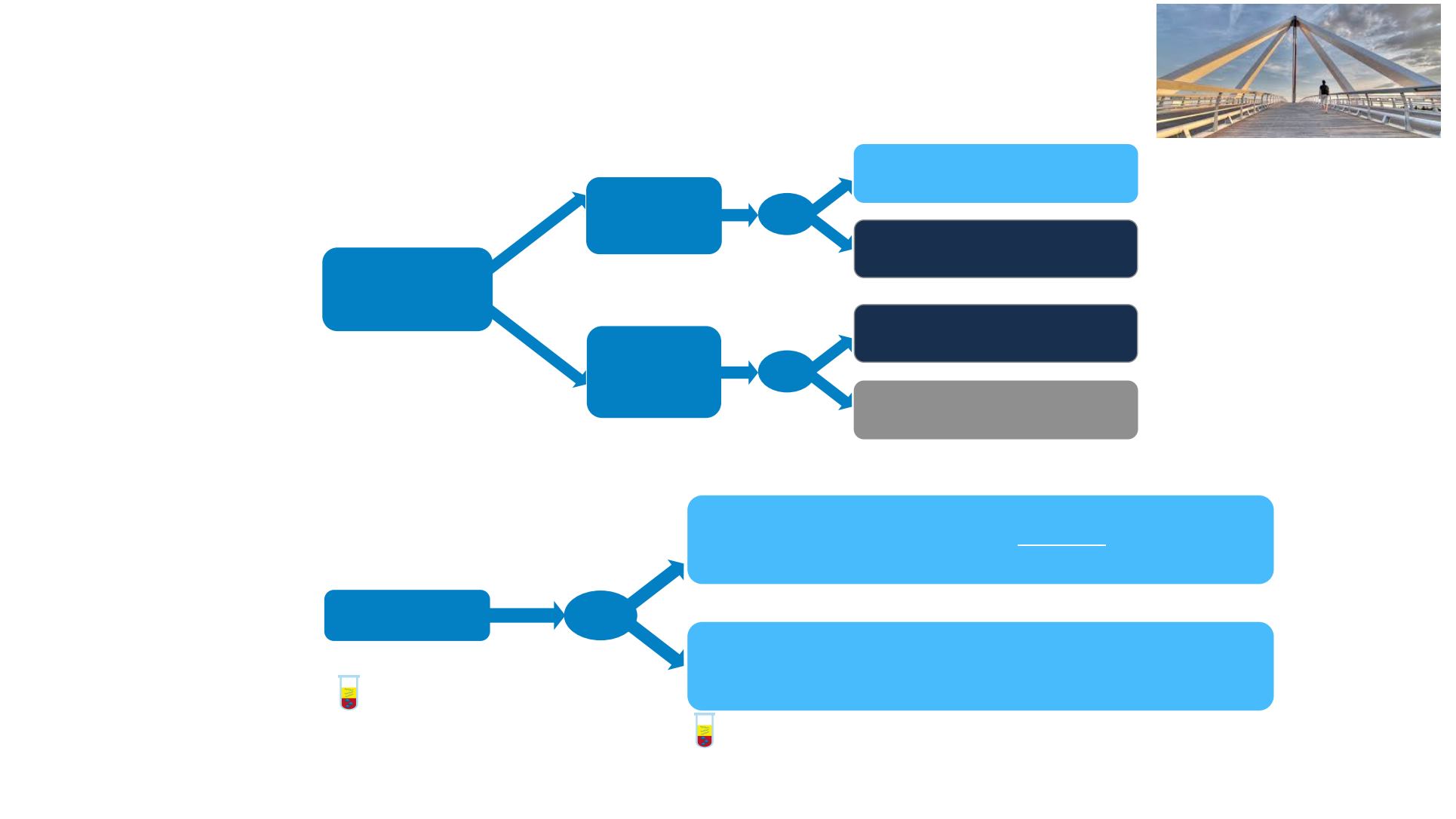
CAIRO5: NCT02162563 (Phase III)
1
Study sponsor: Dutch Colorectal Cancer Group (DCCG).
Liver-limited mCRC
Unresectable
(N ~ 640)
R
WT RAS/
BRAF and
left-sided
primary
MT RAS/
BRAF
and/or
right-sided
primary
R
Panitumumab 6 mg/kg
+ FOLFOX or FOLFIRI
Q2W
Bevacizumab 5 mg/kg
+ FOLFOX or FOLFIRI
Q2W
Bevacizumab
5 mg/kg
+ FOLFOX or FOLFIRI
Q2W
Bevacizumab 5 mg/kg
+ FOLFOXIRI
‡
Q2W
Stratification factors include:
Resectability of liver metastases
(potentially resectable vs
permanently unresectable)
1. Huiskens J, et al. BMC Cancer 2015;15:365; ClinicalTrials.gov identifier: NCT02162563 (accessed 08-03-18)
2. ClinicalTrials.gov identifier: NCT02885753 (accessed 08-03-18)
OSCAR: NCT02885753 (Phase III)
2
Study sponsor: Federation Francophone de Cancerologie Digestive
Liver-limited mCRC
(N
~
268)
Panitumumab 6 mg/kg (WT RAS) or bevacizumab 5 mg/kg (MT RAS) +
oxaliplatin 85 mg/m
2
intra-arterial
+ LV5FU2
†
Q2W
R
Panitumumab 6 mg/kg (WT RAS) or bevacizumab 5 mg/kg (MT RAS) +
oxaliplatin 85 mg/m2 intravenous
+ LV5FU2† Q2W
Treatment until PD or toxicity
Baseline ctDNA
Early response (Day 28) evaluation by ctDNA








