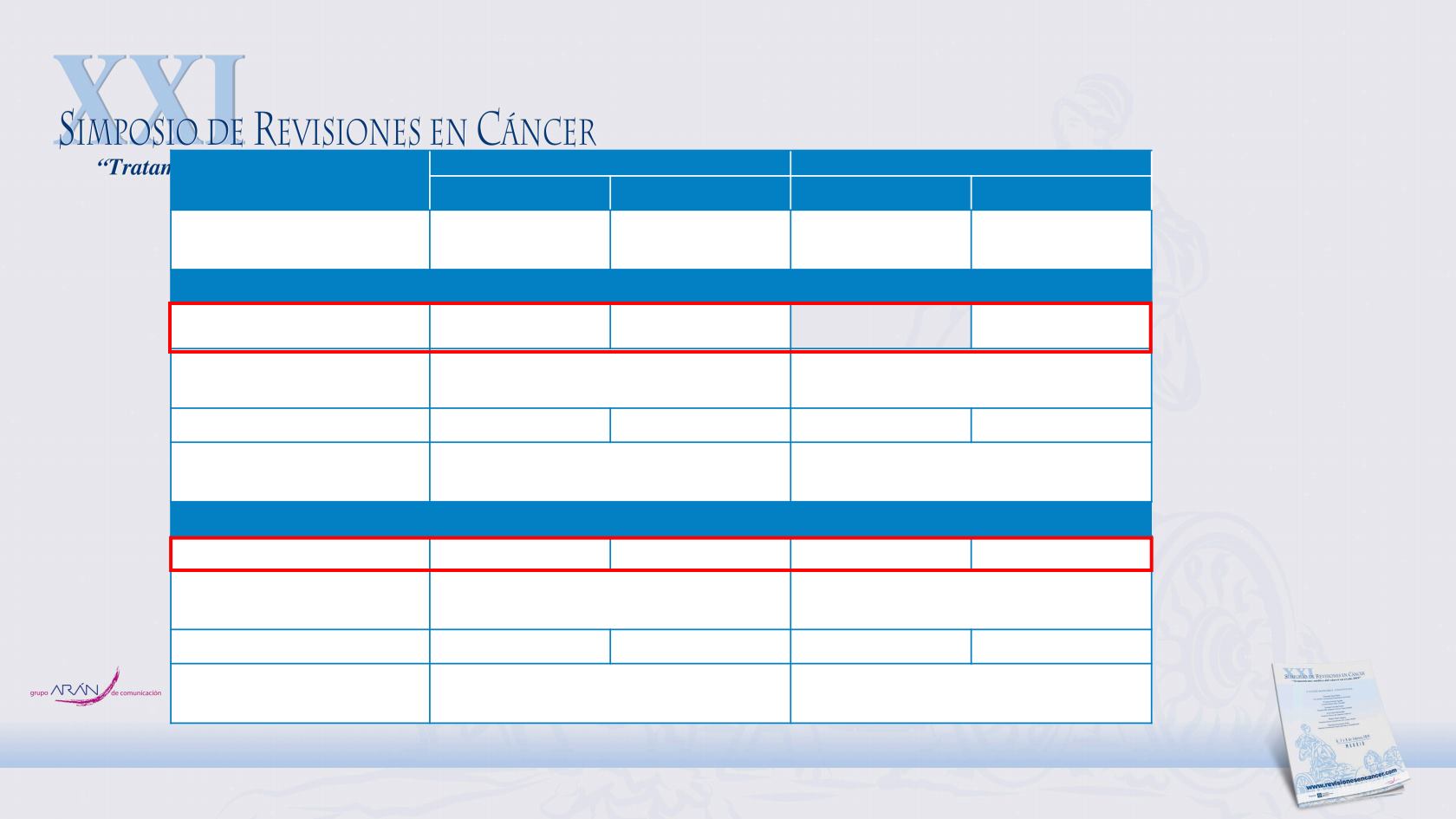
Panitumumab y localizacion tumoral
WT
RAS
/WT
BRAF
PRIME
PEAK
Pmab + FOLFOX FOLFOX Pmab + FOLFOX Bev + FOLFOX
Left, n
Right, n
156
26
148
32
52
13
53
13
Median OS, months
Left
32.5
23.6
43.4
32.0
Adjusted HR
†
(95% CI)
P-value
0.68 (0.52‒0.87)
0.0027
0.76 (0.45‒1.27)
0.2945
Right
22.5
21.5
22.5
23.3
Adjusted HR
†
(95% CI)
P-value
0.97 (0.55‒1.74)
0.9295
0.64 (0.26‒1.58)
0.3326
Median PFS, months
Left
12.9
9.3
14.6
11.5
Adjusted HR
†
(95% CI)
P-value
0.69 (0.54‒0.88)
0.0028
0.65 (0.43‒1.00)
0.0514
Right
8.9
7.3
10.3
12.6
Adjusted HR
†
(95% CI)
P-value
0.75 (0.42‒1.33)
0.3260
0.90 (0.39‒2.07)
0.8092
Boeckx N, et al. Ann Oncol 2017;28:1862-8.
†
For PRIME, adjusted treatment HR was calculated from a model with factors for region and baseline
ECOG PS; for PEAK, adjusted treatment HR was calculated from a model with factors for prior
adjuvant oxaliplatin therapy.
A HR < 1 favours the panitumumab treatment arm.








